结核病(tuberculosis,TB)是一种由结核分枝杆菌(Mycobacterium tuberculosis,M. tuberculosis)引起的慢性传染病。2019年WHO公布2018年全球约有1 000万人罹患结核病,死亡人数高达120万人[1],结核病的防治仍然面临很大的挑战。
结核分枝杆菌作为结核病的病原菌,通过呼吸道进入宿主肺泡巨噬细胞后,能快速并准确地转换其代谢途径来适应宿主内环境,同时利用巨噬细胞等免疫细胞躲避宿主的免疫攻击,导致潜伏感染[2],然而造成结核分枝杆菌潜伏及致病的具体机制至今尚未研究透彻。结核分枝杆菌和鸟分枝杆菌(Mycobacterium avium,M. avium)等致病性分枝杆菌的基因组中大约有10%的开放读码框架(open reading frame,ORF)编码PE/PPE家族蛋白,这些蛋白能够影响菌株的毒力及其与宿主之间的相互作用[3]。例如,针对PPE11的研究发现,其可影响结核分枝杆菌的成膜能力和抗逆环境的能力,与细菌毒力密切相关[4-5]; PE-PGRS30则被证明能抵抗巨噬细胞中的酸性环境,从而维持结核分枝杆菌的胞内生存[6]。Basu等研究发现,PE_PGRS33和PPE34均能结合Toll样受体2(Toll-like receptor 2,TLR2)而激活巨噬细胞和树突细胞,并诱导促进凋亡和坏死的细胞因子释放[7-8]。研究表明,PE/PPEs家族在结核病发病机制中起重要作用。进一步探究其相关机制对于了解结核分枝杆菌的潜伏及开展结核病的防御治疗具有深远的意义。
经结核分枝杆菌与卡介苗(bacillus Calmette-Guérin,BCG)的基因组比对发现,结核分枝杆菌基因组的一些片段在BCG中缺失,此类缺失的片段被称为差异区域(region of differences, RD),RD区被认为可能与结核分枝杆菌的毒力和宿主免疫调节有关[2]。结核分枝杆菌H37Rv菌株RD-11编码5个蛋白,分别为Rv3427c、Rv3428c、Rv3425、Rv3426和Rv3429,其中Rv3425、Rv3426和Rv3429是PPE蛋白家族成员[9]。基因rv3425编码蛋白PPE57(即Rv3425),它是一个潜在的B细胞抗原,可激发体液免疫反应。用重组PPE57蛋白作为包被抗原时,结核病患者与卡介苗接种者的血清阳性率有显著差异,这表明PPE57能有效区分结核病患者与卡介苗接种者,对结核病的诊断有较高应用价值[10]。重组PPE57蛋白联合佐剂免疫小鼠,可诱导脾淋巴细胞产生Th1型免疫反应并高水平分泌γ干扰素(IFN-γ),同时产生高效价的IgG1抗体[11]。PPE57还能结合巨噬细胞TLR2受体,激活下游MAPKs/NF-κB信号通路,显著诱导巨噬细胞分泌促炎症因子〔肿瘤坏死因子(tumor necrosis factor,TNF)-α、白细胞介素(interleukin,IL)-12以及IL-6〕并促进主要组织相容性复合体(major histocompatibility complex,MHC)分子和共刺激分子表达[12],这些研究都显示该蛋白是一种可用于疫苗构建的候选抗原。本研究以耻垢分枝杆菌野生株(Ms)为模式菌株,构建了重组耻垢分枝杆菌Ms-Pact和Ms-Rv3425,并比较它们在菌落形态、成膜能力、疏水性等方面的差异和对逆境的耐受能力; 针对广谱抗生素及结核分枝杆菌特异性药物的抗性,探讨Rv3425蛋白的功能。分析在不同逆境条件下培养的H37Ra中Rv3425内源表达量的变化,并通过细胞和动物实验,观察Rv3425对菌株的胞内存活及毒力的影响。期望本研究结果对结核病的预防和治疗有所帮助。
1 材料和方法 1.1 材料耻垢分枝杆菌与结核分枝杆菌标准株H37Ra为本实验室保藏菌种,质粒Pact、Pact-Rv3425为本实验室保存质粒,THP-1细胞株购于中国科学院细胞库,6~8周龄的BALB/c雄性小鼠购于上海斯莱克实验动物有限责任公司,葡萄糖、氯化钠、乙酰胺、丙三醇、Tween-80、Tween-20、Tris、甘氨酸、PBS、油酸、4%多聚甲醛以及实验室常用抗生素购于生工生物工程(上海)股份有限公司,Middlebrook7H9、Middlebrook7H10培养基购于美国BD公司,牛血清白蛋白(bovine albumin)购于美国AMRESCO公司,利福平、异烟肼、吡嗪酰胺、庆大霉素、过氧化氢酶catalase购于美国Sigma公司,细胞培养基RPM1640、胎牛血清、青/链霉素混合液(100x)购于美国ThermoFisher公司。
1.2 方法 1.2.1 重组耻垢分枝杆菌Ms-Pact、Ms-Rv3425的构建将质粒Pact、Pact-Rv3425经乙醇沉淀纯化后电转进耻垢分枝杆菌Ms感受态中,加入不含Tween-80的7H9-OADC培养基(5% BSA、0.03%过氧化氢酶、2%葡萄糖、0.85%氯化钠、0.01%油酸),37 ℃静置孵育2 h,涂布于含有30 μg/mL卡那霉素的7H10-OADC平板上,待长出菌落挑至7H9-OADC培养基中,37 ℃振荡培养至OD600=0.5时,加入乙酰胺至终浓度34 mmol/L,诱导12 h后行蛋白印迹法验证。
1.2.2 生长曲线的测定将菌株Ms、Ms-Pact、Ms-Rv3425接种于7H9-OADC培养基中,37 ℃振荡培养至对数生长期; 将菌液OD600调成一致,以1:100转接入含有100 mL 7H9-ODAC培养基的锥形瓶中,加入乙酰胺至终浓度34 mmol/L,37 ℃振荡培养; 每隔一段时间取样,梯度稀释涂平板,待长出菌落后进行数板计数。
1.2.3 菌落形态的观察将菌株Ms、Ms-Pact、Ms-Rv3425接种于7H9-OADC培养基中,37 ℃振荡培养至OD600=0.8,加入乙酰胺至终浓度34 mmol/L诱导6 h; 梯度稀释涂板,37 ℃培养箱培养3~5 d; 宏观变倍体显微镜观察并拍照。
1.2.4 聚集能力分析将菌株Ms、Ms-Pact、Ms-Rv3425接种于苏通液体培养基中,加入乙酰胺至终浓度34 mmol/L,37 ℃振荡培养24 h; 于室温静置5 h,比较它们的疏松度并拍照。
1.2.5 成膜能力分析生物膜形成的观察:将菌株Ms、Ms-Pact、Ms-Rv3425接种于7H9-OADC培养基中,37 ℃振荡培养至OD600=0.8;以1:100的比例转接入苏通培养基中,调OD600=0.05;加入乙酰胺至终浓度34 mmol/L,37 ℃静置培养; 分别在第1.5、2、3、5、7、9天观察生物膜的形成及脱落情况并拍照记录。
结晶紫成膜定量实验:将Ms、Ms-Pact、Ms-Rv3425菌株接种于7H9-OADC培养基中,37 ℃振荡培养至OD600=0.8;以1:100的比例转接入苏通培养基中,调OD600=0.05,加入乙酰胺至终浓度34 mmol/L; 转入96孔板,置于37 ℃培养箱培养; 分别在第1.5、2、3、5天弃掉培养基,用无菌水清洗2次; 1%的结晶紫染色液染色30 min,吸掉染色液,并用无菌水清洗至洗涤液接近无色; 加入100%乙醇200 μL/孔,静置1 h; 检测OD570的吸收值。
1.2.6 抗逆性及抗药性分析将培养至对数期的Ms-Pact、Ms-Rv3425菌液调至OD600=1;以1︰50分别转接入pH5.0、pH5.5,0.02%十二烷基磺酸钠(sodium dodecyl sulfate,SDS)、0.1% SDS,750 μg/mL氨苄西林,16 μg/mL、64 μg/mL的利福平/异烟肼的7H9-OADC培养基,加入乙酰胺至终浓度34 mmol/L,37 ℃振荡培养; 培养一定时间后,取菌液稀释涂板,37 ℃静置培养,3 d后进行数板计数。
1.2.7 逆境条件下H37Ra中Rv3425表达量的检测将H37Ra接入7H9-OADC培养基活化至对数期; 以1:100分别转接入pH5.0、pH5.5,5mmol/L H2O2、10 mmol/L H2O2,250 μg/mL氨苄西林、500 μg/mL氨苄西林,0.02% SDS的7H9-OADC培养基并在低氧条件下37 ℃振荡培养H37Ra菌株; 3周后收菌,蛋白印迹法检测Rv3425蛋白的表达。
1.2.8 细胞感染实验复苏并培养THP-1细胞(37 ℃、5%CO2)至状态良好后进行铺板,加入50 ng/mL乙酸肉豆蔻佛波醇(phorbol-12-myristate-13-acetate,PMA)刺激THP-1细胞,连续刺激2~3 d使其分化。将菌液以感染复数(MOI)为10加入细胞感染4 h; 加入20 μg/mL庆大霉素处理2 h,杀死胞外菌; PBS清洗每孔3次,补加1 mL新鲜1640完全培养基; 分别在0、6、18、24 h吸去培养基,每孔加入1 mL 0.025% SDS裂解细胞,梯度稀释涂板; 2~3 d后,待其形成肉眼可见菌落时数板计数。
1.2.9 动物实验选用6周龄的BALB/c雄性小鼠,提前放入动物房适应1周; 将63只小鼠分为4组,其中Ms组20只,Ms-Pact组20只,Ms-Rv3425组20只,PBS组3只; 制作攻毒菌悬液:取乙酰胺诱导后对数生长期的菌20 mL,3 750 rpm室温离心10 min去上清液,收集细菌沉淀物,5 mL PBS洗2次; 加入5 mL PBS重悬菌体,500 rpm离心5 min,收集上层充分悬浮的菌液,用PBS调OD600=0.8(约2×108 CFU/mL)用PBS稀释成所需浓度。尾静脉注射相应菌液100 μL,每只小鼠的攻毒量约为5×107 CFU[13-14],PBS组注射100 μL PBS; 每天观察小鼠的状态及生存状况; 分别于2、7 d,每组小鼠各取5只,对照组1只,颈椎脱臼处死并进行解剖,分离肝脏、脾脏和右肺,研磨组织,10倍梯度稀释,选择合适梯度进行涂板,37 ℃倒置培养,2~3 d后进行菌落计数; 同时取小鼠左肺,用4%的多聚甲醛固定,送至赛维尔公司进行HE染色分析。
1.3 统计学方法所有实验数据均采用Graphpad Prism 5.0软件进行t检验统计分析,P<0.05为差异有统计学意义。
2 结果 2.1 Ms-Rv3425的构建及其表达量鉴定在细菌生长到OD600=0.5时,加入34 mmol/L乙酰胺诱导12 h,收集5 mL菌液,超声破碎获取细菌总蛋白,用anti-Rv3425单克隆抗体进行蛋白印迹法检测。图 1A显示,在相对分子质量(relative molecular mass,Mr)为19 000附近有条带,为目的蛋白Rv3425,表明Ms-Rv3425构建成功。图 1B结果显示,相比于OD600=0.5的诱导时间点以及28 mmol/L乙酰胺的诱导浓度,在Ms-Rv3425生长到OD600=0.8时加入34 mmol/L乙酰胺诱导,Rv3425的表达量最高。由于分枝杆菌内参确定困难,本研究用Bicinchoninic acid(BCA)法对全菌蛋白定量并进行SDS-PAGE,通过考马斯亮蓝染色以确定总蛋白上样量一致,如图 1C。
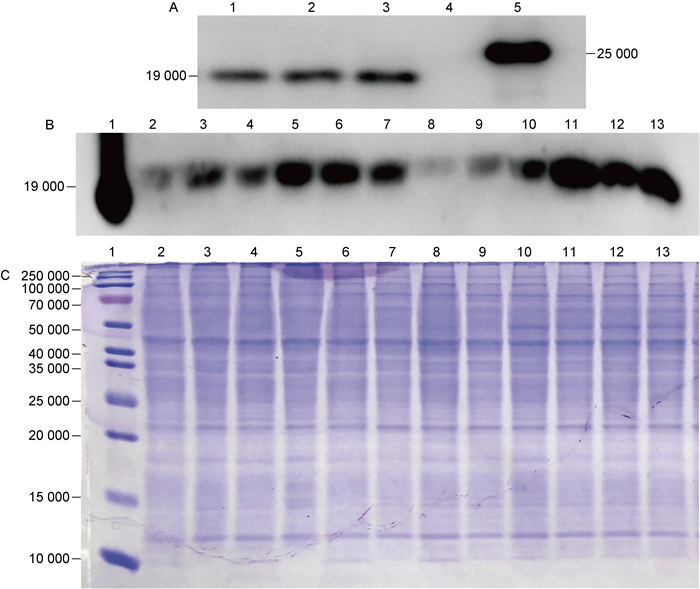
|
| A: Immunolocalization of Rv3425 in recombinant M.smegmatis Ms-Rv3425. 1-3: cell lysate of Ms-Rv3425; 4: cell lysate of Ms-Pact; 5: purified Rv3425. B: Expression levels of Rv3425 in different periods and under different induction conditions. 1: purified Rv3425; 2-4: cell lysate of Ms-Rv3425 induced with 28 mmol/L acetamide at OD600=0.5, at 6 h/12 h/24 h; 5-7: cell lysate of Ms-Rv3425 induced with 28 mmol/L acetamide at OD600=0.8, at 6 h/12 h/24 h; 8-10: cell lysate of Ms-Rv3425 induced with 34 mmol/L acetamide at OD600=0.5, at 6 h/12 h/24 h; 11-13: cell lysate of Ms-Rv3425 induced with 34 mmol/L acetamide at OD600=0.8, at 6 h/12 h/24 h. C: Coomassie blue staining of Ms-Rv3425-induced expression of whole bacteria protein. 1: protein marker; 2-13: are consistent with B2-13. 图 1 重组耻垢分枝杆菌Ms-Rv3425的鉴定与蛋白诱导表达 Fig. 1 Identification and protein-induced expression of recombinant M. smegmatis Ms-Rv3425 |
在不同时间点,取培养的Ms、Ms-Pact、Ms-Rv3425,梯度稀释涂板进行菌落形成单位(colony-forming units,CFU)计数。结果显示,Ms、Ms-Pact和Ms-Rv3425的生长速率无差异,表明外源转入Rv3425不影响耻垢分枝杆菌的体外增殖(图 2)。
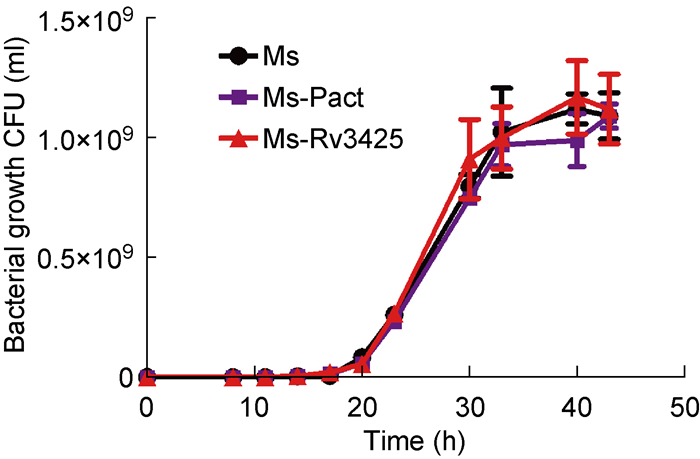
|
| Colony-forming units of Ms, Ms-Pact and Ms-Rv3425 at intervals at 37 ℃, 100 rpm. 图 2 Ms、Ms-Pact、Ms-Rv3425的生长曲线 Fig. 2 Bacterial growth curve of Ms, Ms-Pact, Ms-Rv3425 |
将培养至对数期的3种菌涂布于7H10-OADC平板上,4 d后在宏观变倍体式显微镜下观察菌落形态。结果显示,Ms-Pact和Ms的菌落形态相似,比较扁平,褶皱比较细; 而Ms-Rv3425的菌落整体比较圆润,边缘的晕比较厚,菌落中央高突隆起(图 3A)。
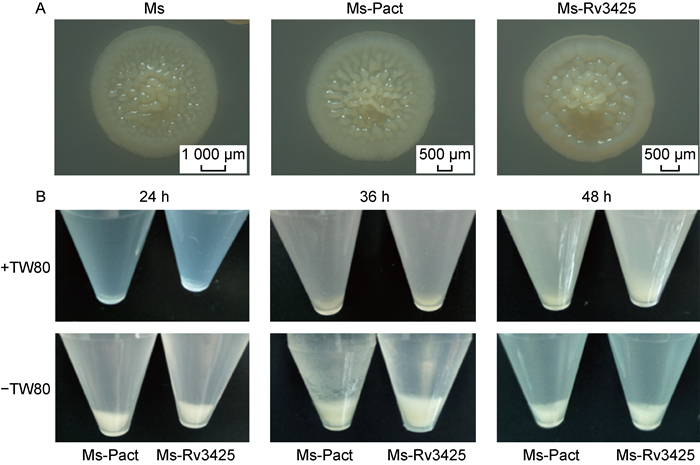
|
| A: Enlarged view of single colony of Ms, Ms-Pact and Ms-Rv3425. B: Aggregate formation by Ms, Ms-Pact and Ms-Rv3425. 图 3 Ms、Ms-Pact、Ms-Rv3425的菌落形态和聚集度 Fig. 3 Colony morphology and aggregation of Ms, Ms-Pact and Ms-Rv3425 |
将菌株培养至对数期后转至静置培养5 h,观察3种菌株聚集度的差异。结果显示,在添加Tween-80和不添加Tween-80的培养基中培养24、36、48 h后,Ms-Pact、Ms-Rv3425的细菌数量几乎是相同的,但是Ms-Rv3425明显更松散,聚集度差(图 3B)。
2.4 外源转入Ms-Rv3425增强了Ms的成膜能力用不含Tween-80的7H9-OADC培养基静置培养Ms、Ms-Pact、Ms-Rv3425,分别在第1、1.5、2、3、4、5、7天记录并分析其成膜情况。结果显示,在第1.5天时,逐渐开始形成生物膜,到第5天菌膜开始脱落。Ms-Rv3425形成生物膜明显早于Ms-Pact,且菌膜面积更大、更厚,褶皱更加明显(图 4A、4B)。此外,分别在第1.5、2、3、5天用结晶紫对菌膜染色行定量分析,结果如图 4C显示,在第1.5、2、3天,Ms-Rv3425成膜量都高于Ms-Pact,P<0.000 5,差异有统计学意义。

|
| A: Pellicle formation in standing cultures of Ms-Pact and Ms-Rv3425 at different times. B: Pellicle formation in standing cultures of Ms, Ms-Pact and Ms-Rv3425 at 48h. C: Quantitative measurement of the biofilm formation by crystal violet staining. ***: P < 0.0005; ****: P < 0.0001. 图 4 3种菌的生物膜形成与成膜量鉴定 Fig. 4 Biofilm formation and crystal violet quantitative experiments |
模拟巨噬细胞的内环境,在0.02% SDS、0.1% SDS,pH5.5、pH5.0这几种逆境条件下培养Ms-Pact和Ms-Rv3425,比较2种菌存活率,分析Rv3425与结核分枝杆菌抵抗逆境的关系。结果显示,Ms-Rv3425抗表面活性剂和耐酸能力显著增强(图 5A)。此外,用含750 μg/mL氨苄西林、16 μg/mL异烟肼、64 μg/mL异烟肼、16 μg/mL利福平、64 μg/mL利福平的7H9-OADC培养基培养上述2种菌,结果发现,Ms-Rv3425对氨苄西林、异烟肼、利福平的耐受性更强(图 5B)。
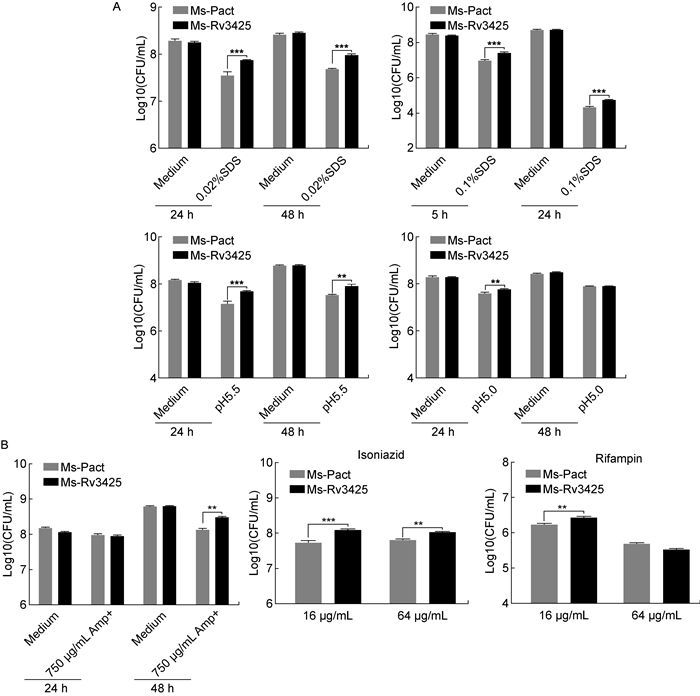
|
| A: Colony-forming units of two strains in presence of 7H9-OADC medium supplemented with 0.02% SDS or 7H9-OADC medium at pH5.5 or 5.0, at 37 ℃, 100 rpm for 24 h and 48 h; Colony-forming units of two strains in presence of 7H9-OADC medium supplemented 0.1% SDS, at 37 ℃, 100 rpm for 5 h and 24 h. B: Colony-forming units of two strains in presence of 7H9-OADC medium supplemented with 750 μg/mL ampicillin, at 37 ℃, 100 rpm for 24 h and 48 h; Colony-forming units of two strains in presence of 7H9-OADC medium supplemented with 16 μg/mL or 64 μg/mL isoniazid, 16 μg/mL or 64 μg/mL Rifampicin, at 37 ℃, 100rpm for 24 h. **: P < 0.005; ***: P < 0.000 5. 图 5 Ms-Pact、Ms-Rv3425的抗逆性与耐药性 Fig. 5 Stress and drug resistance of Ms-Pact, Ms-Rv3425 |
为进一步探讨Rv3425表达量与菌株适应逆境环境之间的关系,在低pH值、低氧、5 mmol/L H2O2、10 mmol/L H2O2、250 μg/mL氨苄西林、500 μg/mL氨苄西林、0.02% SDS条件下培养H37Ra,并检测Rv3425的内源表达量的变化。结果显示,H37Ra菌株在低pH、低氧环境中培养时,Rv3425显著上调; 在250 μg/mL氨苄西林、500 μg/mL氨苄西林、0.02% SDS环境中,Rv3425的表达也略有上调; 而在不同浓度H2O2条件下培养时,Rv3425的内源表达量无显著变化(图 6),这一结果也与上述抗逆实验结果相符合。
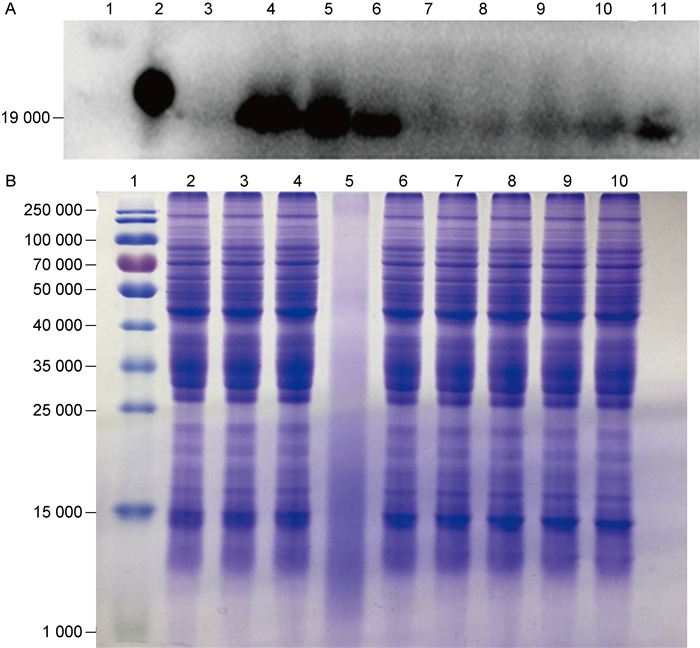
|
| A: Endogenous expression of Rv3425 under adverse conditions. 1: protein marker; 2: purified Rv3425; 3: cell lysate of H37Ra cultured in 7H9-OADC; 4: cell lysate of H37Ra cultured in 7H9-OADC at pH5.5; 5: cell lysate of H37Ra cultured in 7H9-OADC at pH5.0; 6: cell lysate of H37Ra cultured in 7H9-OADC under hypoxic conditions; 7: cell lysate of H37Ra cultured in 7H9-OADC containing 5 mmol/L H2O2; 8: cell lysate of H37Ra cultured in 7H9-OADC containing 10 mmol/L H2O2; 9: cell lysate of H37Ra cultured in 7H9-OADC containing 250 μg/mL Amp+; 10: cell lysate of H37Ra cultured in 7H9-OADC containing 500 μg/mL Amp+; 11: cell lysate of H37Ra cultured in 7H9-OADC containing 0.02% SDS. B: Coomassie blue staining of H37Ra whole bacteria protein under stress. 1: protein marker; 2-10: are consistent with A 3-11. 图 6 逆境条件下Rv3425的内源表达量 Fig. 6 Endogenous expression of Rv3425 under adverse conditions |
THP-1细胞感染实验结果显示,Ms-Rv3425组的侵染效率最高,在胞内存活能力也显著强于Ms及Ms-Pact组(图 7A)。BALB/c小鼠攻毒实验结果也显示,外源转入Rv3425增强了耻垢分枝杆菌的毒性,如图 7B所示,Ms-Rv3425组的小鼠从第2天开始死亡,到第20天共死亡7只; Ms-Pact组小鼠共死亡4只; Ms组小鼠则无死亡。Ms-Rv3425组与Ms-Pact组相比,P=0.016,差异有统计学意义。对于脾、肺、肝3种脏器的载菌量检测结果显示,在脾和肺中,Ms-Rv3425组与Ms-Pact组差异不显著(图 7C)。各脏器的病理切片结果如图 7D所示,Ms-Rv3425组小鼠的脏器病变程度要显著强于Ms组和Ms-Pact组,Ms-Rv3425组肝窦轻度淤血,扩张程度大,伴有大量胞核固缩深染,且有多处炎症细胞灶性浸润; 脾脏中多核巨细胞增多,红髓和白髓界限不清,红髓减少,白髓增多,脾小结结构不清,出现明显的炎症反应; 肺中也出现了明显的组织浸润。细胞感染与小鼠攻毒实验的结果基本一致,表明外源转入Rv3425能够显著增强耻垢分枝杆菌的毒力。
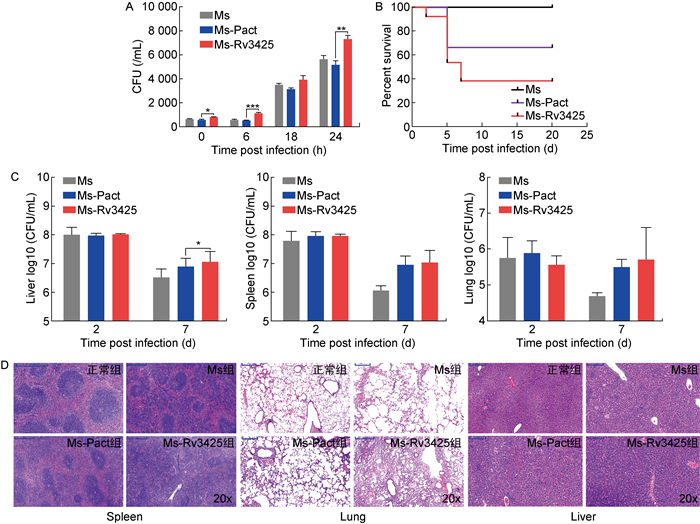
|
| A: Colony-forming units of infected THP-1 cells at 0 h, 6 h, 18 h, 24 h. *: P < 0.05; **: P < 0.01; ***: P < 0.001. B: Survival curve of infected mice. C: Colony-forming units determined in lysates of lung, spleen, and liver from infected mice. D: From left to right, hematoxylin-stained spleen, lung, and liver sections of infected mice. 图 7 THP-1细胞感染与BALB/c小鼠感染结果 Fig. 7 THP-1 cell infection and BALB/c mouse infection |
分枝杆菌属具有复杂菌落形态和生物膜,与毒力相关缺乏生物膜的鸟分枝杆菌和海分枝杆菌的转座子突变体在耐药性及细胞侵染能力方面都有所减弱[15-17]。本研究发现Ms-Rv3425与Ms及Ms-Pact相比,菌落形态更加粗糙隆起,生物成膜能力更强,耐胁迫、抗异烟肼等结核药物的能力显著上升。此外,H37Ra在低氧、低pH值等压力条件下Rv3425的内源表达量显著上调。结核分枝杆菌细胞壁厚,与细胞壁核心共价结合的霉菌酸和游离脂质形成高度不透水外膜赋予了分枝杆菌一定的抗逆性和耐药性[18-19]。
结核分枝杆菌通过胞吞作用进入巨噬细胞,其聚集会激活巨噬细胞,使胞内酸性逐渐增强,pH值由6.2逐渐下降至5.5[20]。结核分枝杆菌通过长期的进化,对这种酸性环境产生了一定的耐受性,其耐酸的能力被认为与结核分枝杆菌的毒力存在关联[21-22]。细胞感染实验结果显示,Rv3425可以增强耻垢分枝杆菌在巨噬细胞内的存活能力。动物实验中,由于耻垢分枝杆菌的毒力较小,试验选取遗传背景为近交系、免疫能力较差的BALB/c雄性小鼠,通过尾静脉注射的方式进行感染。小鼠的生存曲线、各脏器载菌量以及脏器病变程度结果显示,外源转入Rv3425可增强耻垢分枝杆菌的毒力。
因此,从本研究结果推测,外源转入的Rv3425可能是通过改变分枝杆菌细胞壁结构或脂质组分影响其菌落形态和生物膜等表型,并降低细胞壁的通透性,从而提高细菌的抗逆性和抗药性。后续还需通过薄层色谱分析(thin layer chromatography,TLC)和气相色谱-质谱联用(gas chromatography-mass spectrometry,GC-MS)对细菌脂质、脂肪酸进行更为详细的分析以揭示有关机制。希望通过深入研究Rv3425的功能及相关机制为其应用于结核病的免疫学诊断及疫苗构建提供理论依据。
| [1] |
World Health Organization. Global Health Observatory (GHO) data: Tuberculosis (TB)[DB/OL]. Geneva: World Health Organization, 2018. http://www.who.int/gho/tb.
|
| [2] |
Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, Ginsberg A, Swaminathan S, Spigelman M, Getahun H, Menzies D, Raviglione M. Tuberculosis[J]. Nat Rev Dis Primers, 2016, 2: 16076.
[DOI]
|
| [3] |
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence[J]. Nature, 1998, 393(6685): 537-544.
[DOI]
|
| [4] |
Singh P, Rao RN, Reddy JR, Prasad RB, Kotturu SK, Ghosh S, Mukhopadhyay S. PE11, a PE/PPE family protein of Mycobacterium tuberculosis is involved in cell wall remodeling and virulence[J]. Sci Rep, 2016, 6: 21624.
[DOI]
|
| [5] |
Rastogi S, Singh AK, Pant G, Mitra K, Sashidhara KV, Krishnan MY. Down-regulation of PE11, a cell wall associated esterase, enhances the biofilm growth of Mycobacterium tuberculosis and reduces cell wall virulence lipid levels[J]. Microbiology, 2017, 163(1): 52-61.
[URI]
|
| [6] |
Iantomasi R, Sali M, Cascioferro A, Palucci I, Zumbo A, Soldini S, Rocca S, Greco E, Maulucci G, de Spirito M, Fraziano M, Fadda G, Manganelli R, Delogu G. PE_PGRS30 is required for the full virulence of Mycobacterium tuberculosis[J]. Cell Microbiol, 2012, 14(3): 356-367.
[DOI]
|
| [7] |
Bansal K, Sinha AY, Ghorpade DS, Togarsimalemath SK, Patil SA, Kaveri SV, Balaji KN, Bayry J. Src homology 3-interacting domain of Rv1917c of Mycobacterium tuberculosis induces selective maturation of human dendritic cells by regulating PI3K-MAPK-NF-kappaB signaling and drives Th2 immune responses[J]. J Biol Chem, 2010, 285(47): 36511-36522.
[DOI]
|
| [8] |
Basu S, Pathak SK, Banerjee A, Pathak S, Bhattacharyya A, Yang Z, Talarico S, Kundu M, Basu J. Execution of macrophage apoptosis by PE_PGRS33 of Mycobacterium tuberculosis is mediated by Toll-like receptor 2-dependent release of tumor necrosis factor-alpha[J]. J Biol Chem, 2007, 282(2): 1039-1050.
[DOI]
|
| [9] |
Wang S, Chen J, Zhang Y, Diao N, Zhang S, Wu J, Lu C, Wang F, Gao Y, Shao L, Jin J, Weng X, Zhang W. Mycobacterium tuberculosis region of difference (RD) 2 antigen Rv1985c and RD11 antigen Rv3425 have the promising potential to distinguish patients with active tuberculosis from M. bovis BCG-vaccinated individuals[J]. Clin Vaccine Immunol, 2013, 20(1): 69-76.
[URI]
|
| [10] |
Wang J, Qie Y, Zhang H, Zhu B, Xu Y, Liu W, Chen J, Wang H. PPE protein (Rv3425) from DNA segment RD11 of Mycobacterium tuberculosis: a novel immunodominant antigen of Mycobacterium tuberculosis induces humoral and cellular immune responses in mice[J]. Microbiol Immunol, 2008, 52(4): 224-230.
[DOI]
|
| [11] |
Zhang H, Wang J, Lei J, Zhang M, Chen Y, Wang H. PPE protein (Rv3425) from DNA segment RD11 of Mycobacterium tuberculosis: a potential B-cell antigen used for serological diagnosis to distinguish vaccinated controls from tuberculosis patients[J]. Clin Microbiol Infect, 2007, 13(2): 139-145.
[DOI]
|
| [12] |
Xu Y, Yang E, Huang Q, Ni W, Kong C, Liu G, Li G, Su H, Wang H. PPE57 induces activation of macrophages and drives Th1-type immune responses through TLR2[J]. J Mol Med (Berl), 2015, 93(6): 645-662.
[DOI]
|
| [13] |
Liu D, Hao K, Wang W, Peng C, Dai Y, Jin R, Xu W, He L, Wang H, Wang H, Zhang L, Wang Q. Rv2629 overexpression delays Mycobacterium smegmatis and mycobacteria tuberculosis entry into log-phase and increases pathogenicity of Mycobacterium smegmatis in mice[J]. Front Microbiol, 2017, 8: 2231.
[DOI]
|
| [14] |
Shleeva MO, Kondratieva TK, Demina GR, Rubakova EI, Goncharenko AV, Apt AS, Kaprelyants AS. Overexpression of adenylyl cyclase encoded by the Mycobacterium tuberculosis Rv2212 gene confers improved fitness, accelerated recovery from dormancy and enhanced virulence in mice[J]. Front Cell Infect Microbiol, 2017, 7: 370.
[DOI]
|
| [15] |
Kansal RG, Gomez-Flores R, Mehta RT. Change in colony morphology influences the virulence as well as the biochemical properties of the Mycobacterium avium complex[J]. Microb Pathog, 1998, 25(4): 203-214.
[DOI]
|
| [16] |
Yamazaki Y, Danelishvili L, Wu M, Hidaka E, Katsuyama T, Stang B, Petrofsky M, Bildfell R, Bermudez LE. The ability to form biofilm influences Mycobacterium avium invasion and translocation of bronchial epithelial cells[J]. Cell Microbiol, 2006, 8(5): 806-814.
[DOI]
|
| [17] |
Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis[J]. Lancet, 2003, 362(9387): 887-899.
[DOI]
|
| [18] |
Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure[J]. Proc Natl Acad Sci USA, 2008, 105(10): 3963-3967.
[DOI]
|
| [19] |
Zuber B, Chami M, Houssin C, Dubochet J, Griffiths G, Daffé M. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state[J]. J Bacteriol, 2008, 190(16): 5672-5680.
[DOI]
|
| [20] |
Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, Cosma CL, Ramakrishnan L. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids[J]. Nature, 2014, 505(7482): 218-222.
[DOI]
|
| [21] |
Abramovitch RB, Rohde KH, Hsu FF, Russell DG. aprABC: a Mycobacterium tuberculosis, complex-specific locus that modulates pH-driven adaptation to the macrophage phagosome[J]. Mol Microbiol, 2011, 80(3): 678-694.
[DOI]
|
| [22] |
Giotis ES, McDowell DA, Blair IS, Wilkinson BJ. Role of branched-chain fatty acids in pH stress tolerance in Listeria monocytogenes[J]. Appl Environ Microbiol, 2007, 73(3): 997-1001.
|
 2020, Vol. 15
2020, Vol. 15


