2. 国家老年疾病临床医学研究中心(复旦大学附属华山医院),上海 200040
2. National Clinical Research Center on Aging and Medicine, Huashan Hospital, Shanghai 200040, China
乙型肝炎病毒(hepatitis B virus,HBV)的持续性感染可造成慢性乙型肝炎(chronic hepatitis B,CHB),后期发展为致命性肝病。全球约有2.6亿慢性HBV感染者,其中约88.7万人死于HBV相关的肝硬化、肝细胞癌(hepatocellular carcinoma, HCC)等终末期肝脏疾病[1]。肝脏是脂质代谢的必要器官,而肝脏脂质代谢失衡会导致异常的脂质积累,引起肝细胞脂肪变性,进而发展为严重肝脏疾病[2]。
HBV属于正嗜肝DNA病毒属,其基因组约有3 200个碱基对。HBV基因组含有4个开放阅读框,可合成9种病毒蛋白。HBV的包膜蛋白,包括大蛋白、中蛋白、小蛋白,统称为乙型肝炎病毒表面抗原(hepatitis B virus surface antigen,HBsAg)。当HBV DNA整合到宿主细胞基因组中后,即使HBV复制水平很低,HBsAg也仍可持续表达[3]。HBsAg的持续存在不仅是乙型肝炎慢性化的标志,也是重要的致病因素。肝内HBsAg的大量表达与严重肝脏疾病进展相关[4-6]。此外,有研究表明,血清HBsAg水平和持续时间与宿主免疫耐受状态呈正相关性,高水平的HBsAg使机体无法产生有效的免疫应答[7]。HBsAg能与宿主来源的脂质装配组成亚病毒颗粒。在HBV持续性感染中,亚病毒颗粒数量远远超过完整的病毒颗粒,可达后者1 000倍以上。亚病毒颗粒本质上是一种脂蛋白,其脂质与蛋白质之比约为0.35,这些颗粒的形成需要大量宿主来源的脂质[8]。因此,CHB患者体内持续性产生的大量亚病毒颗粒需要不断消耗肝细胞脂质,可引起肝脏脂质代谢异常。
近年来,HBV X蛋白(HBV X protein,HBx)、核心蛋白调节肝细胞脂质代谢的多种机制时有报道[9-12],但关于HBsAg与宿主脂质代谢调控的研究较少。此外,关于HBV持续性感染与肝脏脂质代谢之间关系的临床数据与体外研究、动物模型结果均存在矛盾。一项队列研究表明,与HBsAg阴性者相比,HBsAg阳性者发生非酒精性脂肪肝(nonalcoholic fatty liver disease,NAFLD)的风险更低[13, 14],而涉及的机制尚不明确。本研究对稳定表达HBsAg的HepG2-S-G2细胞系及其对照细胞系HepG2-neo-F4进行转录组学分析,探讨HBsAg对脂质代谢的调控机制,期望对HBV持续性感染与肝脏脂质代谢的关系研究提供数据。
1 材料和方法 1.1 材料 1.1.1 细胞系HepG2.2.15细胞系:稳定转染含4个5′-3′串联的HBV基因组的质粒pDolTHBV-1入HepG2细胞系[15],购自美国典型微生物菌种保藏中心。
HepG2-neo-F4细胞系:将空载质粒pCMV-script转染HepG2细胞,构建成稳定转染细胞系,本课题组构建[16]。
HepG2-S-G2细胞系:将C型HBV分离株C8[17]中的SHBs基因克隆片段插入pCMV-script质粒,构建成pCMV-S质粒。将pCMV-S质粒转染HepG2细胞,构建成稳定转染细胞系,本课题组构建[16]。
3种稳定转染细胞系均在遗传霉素(geneticin,G418)筛选下传代培养,并置于液氮保存。
1.1.2 培养基DMEM培养基购自美国GIBCO公司,胎牛血清购自美国CORNING公司,G418购自加拿大WISENT公司。
1.1.3 试剂辛伐他汀、洛伐他汀及油红O染色液(培养细胞专用)均购自上海源叶生物科技有限公司。TRIZOL购自美国Invitrogen公司,反转录试剂盒EastepⓇRT Master Mix Kit购自上海普洛麦格生物产品有限公司,GoTaqⓇqPCR Master Mix购自美国Promega公司。游离脂肪酸(non-esterified fatty acids,NEFA)检测试剂盒和总胆固醇(total cholesterol,TCHO)检测试剂盒均购自南京建成生物工程研究所。HBsAg ELISA检测试剂盒购自上海科华生物有限公司,二甲基亚砜(dimethyl sulfoxide,DMSO)(≧99.7%)购自美国Sigma公司,细胞计数试剂盒(Cell Counting Kit-8,CCK-8)购自日本东仁化学公司,3-酮酸辅酶A转移酶1(3-oxoacid CoA-transferase 1,OXCT1)抗体和细胞色素氧化酶P450家族4亚家族F成员3(cytochrome P450 family 4 subfamily F member 3,CYP4F3)抗体均购自美国Proteintech公司,β-actin抗体购自上海Abmart公司,山羊抗兔抗体购自北京康为世纪生物科技有限公司。
1.1.4 引物OXCT1[18]和CYP4F3[19]荧光定量聚合酶链反应(polymerase chain reaction,PCR)引物由华大基因科技有限公司合成,引物序列见表 1。
PCR仪购自德国Eppendorf公司,WB电泳槽和凝胶成像仪购自美国Bio-Rad公司。
1.2 方法 1.2.1 细胞培养所用细胞均在含有10%胎牛血清、200 μg/mL G418培养基中,置37 ℃、5% CO2条件下培养。
1.2.2 转录组测序与分析将培养HepG2-S-G2细胞和HepG2-neo-F4细胞的12孔板中的培养基吸弃后,加入适量磷酸盐缓冲液(phosphate buffer saline,PBS)清洗2遍。加入TRIZOL,用移液器反复吹打孔内细胞20~30次(冰上操作),同组的3孔细胞均转移入同一预冷1.5 mL离心管,混合均匀,迅速转移入-80 ℃冰箱保存或直接抽提细胞RNA。
采用Agilent Bioanalyzer 2100进行质检,QubitⓇ3.0 Fluorometer和NanoDrop One定量总RNA。RNA样本量、纯度、完整性均合格,方可进行后续操作。构建paired-end文库,即用poly-T寡聚磁珠纯化含有poly-A的mRNA。纯化后的mRNA在94 ℃下8 min解离成小片段,反转录合成第1链cDNA。利用DNA聚合酶和RNA酶H合成第2链cDNA。cDNA经过末端修复后,3′末端添加单个碱基A和连接接头。然后通过PCR对产物进行纯化和富集,创建最终的测序样本cDNA文库,并对纯化后的文库进行定量。使用Agilent Bioanalyzer 2100进行验证,以确定插入物的大小并计算摩尔浓度。依照cBot User Guide的标准流程,利用稀释至10 pmol/L的文库完成簇生成。在Illumina NovaSeq6000(Illumina,USA)上测序并进行序列比对。计算出表征基因表达定量的FPKM(fragments perkilobase million)值,以用于不同样本间的横向比较。对样本组间基因表达进行差异分析,计算得到P值后进行多重假设检验校正,校正后的P值被称为q值。同时,根据FPKM值计算差异表达倍数(fold change,FC),常用log2(FC)来表示。本次差异基因筛选标准为:q值< 0.05且FC>2,筛选差异基因并绘制差异基因分布图。基于京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)数据库(http://deweylab.github.io/RSEM/, https://github.com/TransDecoder),对上述筛选到的差异基因进行KEGG富集分析,找到显著富集的相关通路。q值< 0.05被认为差异具有统计学意义。
1.2.3 荧光定量PCR检测差异基因OXCT1和CYP4F3的mRNA表达TRIZOL法抽提HepG2-S-G2细胞和HepG2-neo-F4细胞的总RNA,用EastepⓇRT Master Mix Kit试剂盒进行反转录,合成第1链cDNA,用GoTaqⓇqPCR Master Mix试剂盒(实时荧光定量PCR法)对OXCT1和CYP4F3基因的mRNA表达量进行检测。OXCT1和CYP4F3的实时PCR引物见表 1。实时PCR的反应条件为95 ℃ GoTaqⓇ Hot Start聚合酶激活2 min;95 ℃变性15 s,60 ℃退火延伸1 min,共40个循环。管家基因β-actin作为内参,actin的引物见表 1。
1.2.4 蛋白质印迹法检测差异基因OXCT1和CYP4F3的蛋白表达裂解细胞蛋白并用二喹啉甲酸(bicinchoninic acid, BCA)法检测蛋白浓度后,加入5×loading buffer,100 ℃恒温煮沸蛋白约10 min,微离心后使用WB检测蛋白表达。
上样后,样品在SDS-PAGE浓缩胶中以80 V恒压电泳30 min,待蛋白迁移至10% SDS-PAGE分离胶时,调高电压至110 V恒压,继续电泳约1.5 h。用聚偏二氟乙烯膜(polyvinylidene fluoride,PVDF)膜进行湿转,280 mA恒流1.5 h。将有蛋白印迹的PVDF膜浸入5%(w/v)脱脂奶粉封闭2 h。将膜放入孵育液,一抗孵育4 ℃过夜,磷酸盐吐温缓冲液(phosphate buffer saline with tween,PBST)或三羟甲基氨基甲烷盐酸盐吐温缓冲液(tris buffered saline tween,TBST)洗膜。适当稀释辣根过氧化物酶标记的二抗,置室温避光孵育1 h。再次洗膜后,将膜正面朝上,置于凝胶成像系统Image Lab 3.0软件进行曝光显色。
1.2.5 油红O染色观察HepG2-S-G2细胞的脂质水平37 ℃、5% CO2条件下分别培养HepG2-S-G2和HepG2-neo-F4细胞48~72 h。PBS清洗后取固定液Cell ORO Fixative固定细胞15~25 min,稍冲洗,晾干后滴加油红O染色液(ORO Stain A: ORO Stain B以3∶2比例混合,静置约10 min,现用现配),染色10~15 min。稍冲洗后加入Mayer苏木素染色液复染细胞核1~2 min,显微镜下观察。
1.2.6 细胞游离脂肪酸与总胆固醇检测裂解细胞蛋白并检测蛋白浓度,每个蛋白样品设置5~7个重复孔,分别按照NEFA和TCHO试剂盒说明书进行检测。在96孔板的空白孔、校准孔、样本孔中分别加入双蒸水、标准品、待测样本后,再加入工作液,混匀后,置37 ℃恒温孵育5 min/10 min,酶标仪读数。
1.2.7 细胞降脂处理后检测HBsAg将HepG2.2.15细胞传代并均匀接种至96孔细胞培养板,37 ℃、5% CO2条件下培养6 h后,将培养液更换成无血清DMEM,饥饿处理12~18 h。将辛伐他汀、洛伐他汀分别溶于DMSO,各自配制成1 μmol/L和5 μmol/L两种浓度的溶液;对照组为等量DMSO处理,并在24 h撤去旧培养基,换成对应药物浓度的新培养基。培养48 h后,ELISA试剂盒检测细胞上清液中的HBsAg及CCK8检测细胞增殖。样品适当稀释后,根据标准曲线计算HBsAg含量(半定量)。利用CCK8的检测结果对各组细胞进行均一化处理后,计算各组细胞HBsAg分泌量与对照组细胞的比值。
1.3 统计学分析利用Graphpad Prism 6.02软件对实验数据进行两样本差异显著性的t检验(除转录组学分析外),进行统计学分析。P < 0.05被认为差异具有统计学意义,P>0.05时认为差异无统计学意义。
2 结果 2.1 细胞转录组筛选到脂质代谢差异基因与富集通路为排除机体多种复杂因素的影响,针对性研究HBsAg与宿主细胞脂质代谢之间的关系,选用稳定持续性表达HBsAg的细胞系HepG2-S-G2及其对照细胞系HepG2-neo-F4,通过对两种细胞转录组的对比分析,筛选出515个差异基因,结果如图 1。
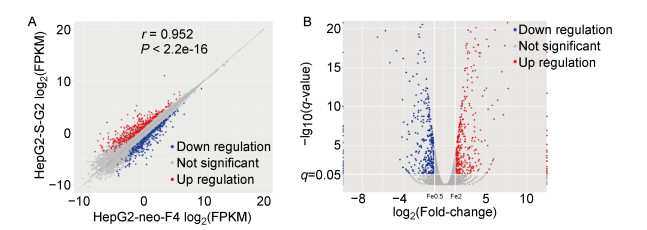
|
| A: X-axis represents the gene expression abundances of HepG2-neo-F4 and Y-axis represents the gene expression abundances of HepG2-S-G2 cells. B: Log2 (Fold-change) represents the fold change of differential genes expression.-Lg10 (q-value) indicates the degree of the difference. q-value < 0.05 and | log2 (fold change) | >1 were all differential genes. Red indicates the up-regulated differential genes of cells, and blue indicates the down regulated differential genes of cells in A and B. 图 1 HepG2-S-G2与HepG2-neo-F4细胞差异基因的转录表达分布 Fig. 1 The scatter plots of differential genes transcription in the HepG2-S-G2 and HepG2-neo-F4 cells |
经KEGG分析发现,在“代谢”这一功能分类中,除全局和概览通路(global and overview maps)外,差异基因数目最多的是脂质代谢通路,如图 2A。KEGG富集到243条通路,通过进一步分析脂质代谢中的次级通路富集发现,脂肪酸合成通路的富集程度最高,即富集因子最高;其次为初级胆汁酸合成通路、亚油酸代谢途径,如图 2B。
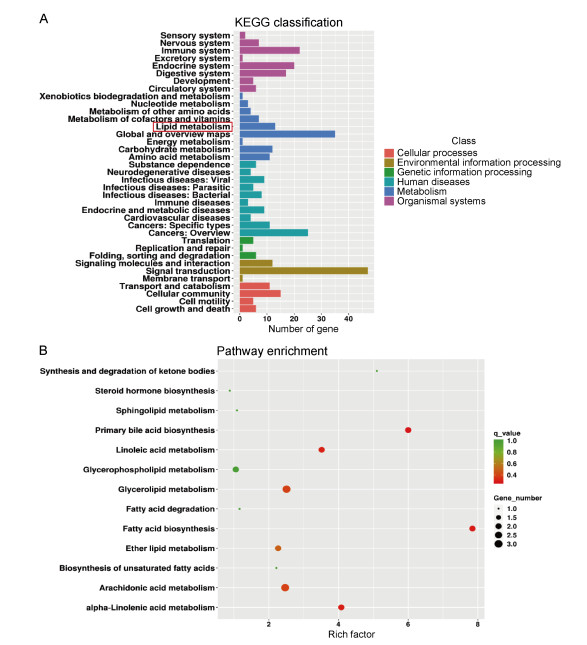
|
| A: X-axis represents the number of differential genes, and Y-axis represents the function annotation of class 2 under the six classifications of KEGG database. The red box is the focus of this study. B: The enrichment pathway in lipid metabolism. Combined with X-axis, the secondary lipid metabolism pathway with different enrichment degree can be found in Y-axis. 图 2 细胞差异基因在KEGG数据库中的脂质代谢及其次级通路的功能分布 Fig. 2 The functional distribution of lipid metabolism and its secondary enrichment pathways of cellular differential genes in the KEGG database |
为明确脂质代谢通路中差异基因的作用,分析脂质代谢通路中筛选发现的13个差异基因,其中8个表达基因上调、5个表达基因下调,均为显著差异表达,变化范围在2~10倍,如图 3。

|
| The differential genes involved in lipid metabolism in the histogram. Y-axis represents the fold change of differences, and X-axis represents the names of the differential genes. Orange bars represent up-regulated genes, while green bars represent down regulated genes. #0.01 < q-value < 0.05; ## 0.001 < q-value < 0.01; ###0.000 1 < q-value < 0.001; ####q-value < 0.000 1. 图 3 参与脂质代谢通路的差异基因 Fig. 3 The differential genes involved in lipid metabolism pathways |
结合脂质代谢通路差异基因的分析发现,参与脂肪酸合成通路的2个差异基因转录水平均被上调,即长链脂酰辅酶A合成酶1(acyl-CoA synthetase long chain family member,ACSL1)基因和线粒体3-酮脂酰-酰基载体蛋白合成酶(3-oxoacyl-acyl carrier protein synthase, mitochondrial,OXSM)基因。结合KEGG数据库中这些基因表达蛋白通路中作用来看,作为脂肪酸合成的关键酶,ACSL1和OXSM的显著上调可促进长链脂肪酸的从头合成。胆汁酸辅酶A:氨基酸N-酰基转移酶(bile acid-CoA: amino acid N-acyltransferase,BAAT)、人分泌磷脂酶A2-IIA(phospholipase A2 group ⅡA,PLA2G2A)和脂肪特异性磷脂酶A2第16型(phospholipase A2 type 16,PLA2G16)基因表达上调可促进不饱和脂肪酸生成;而细胞色素氧化酶P450家族4亚家族F成员3(cytochrome P450 family 4 subfamily F member 3,CYP4F3)基因表达下调可减少花生四烯酸等不饱和脂肪酸进一步代谢。肝型脂肪酶C(lipase C,LIPC)基因表达上调可以促进脂肪酸和胆固醇合成,二磷酸尿苷葡糖醛酸转移酶家族2成员A3(uridine diphosphate glucuronosyltransferase family 2 member A3,UGT2A3)下调可以减少胆固醇向类固醇激素的转化。根据以上脂质代谢差异基因表达的关键酶主要参与脂肪酸和胆固醇代谢过程的提示,推测了HBsAg引起细胞脂肪酸和胆固醇等脂质合成增强、消耗分解减少等代谢变化的潜在机制,如图 4。
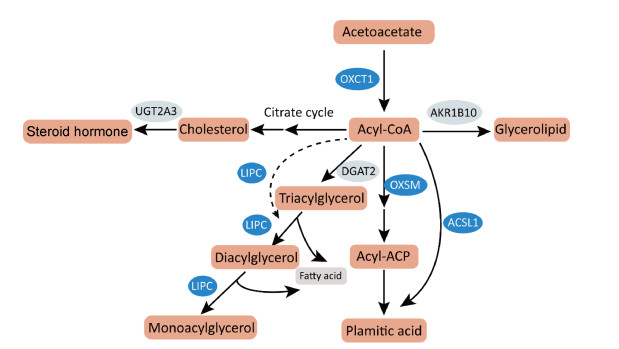
|
| Long bars represent related products in lipid metabolism. Elliptical frames represent some differential key enzymes, among which blue represents up-regulated gene and light color represents down regulated gene. The solid arrow represents direct action, and the dotted arrow represents indirect action. 图 4 HBsAg调控细胞脂质代谢变化的潜在机制 Fig. 4 The potential mechanisms of HBsAg regulating cellular lipid metabolism |
脂质代谢通路差异基因分析提示,与对照细胞相比,HepG2-S-G2细胞转录组中OXCT1基因转录水平上调约10倍,CYP4F3基因下调为对照细胞的1/4左右。为检测HepG2-S-G2、HepG2-neo-F4细胞转录组中筛选分析出的脂质代谢差异基因的可信度,使用实时PCR对上述两个基因进行检测。结果提示,与HepG2-neo-F4细胞相比,HepG2-S-G2细胞的OXCT1 mRNA水平升高约9倍(图 5A),与转录组测序结果基本一致;HepG2-S-G2细胞的CYP4F3转录水平相对下调,与细胞转录组分析结果相符(图 5B)。
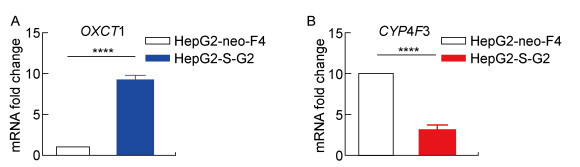
|
| A: The OXCT1 mRNA expression of HepG2-S-G2 is significantly higher than that of HepG2-neo-F4. B: The CYP4F3 mRNA expression of HepG2-S-G2 is significantly lower than that of HepG2-neo-F4. ****: P < 0.000 1. 图 5 HepG2-S-G2细胞与HepG2-neo-F4细胞中OXCT1、CYP4F3 mRNA表达差异 Fig. 5 The differential mRNA expression of OXCT1 and CYP4F3 in the HepG2-S-G2 and HepG2-neo-F4 cells |
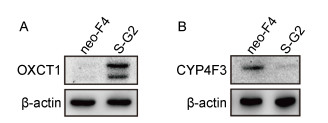
为明确转录水平的差异基因是否在蛋白水平发生相应变化,以实现对后续脂质代谢的调控,利用WB检测OXCT1和CYP4F3的蛋白表达。结果如图 6所示,OXCT1和CYP4F3蛋白均出现相应的显著上调、下调表达,并且趋势与转录组分析一致。
|
| A: The OXCT1 protein of HepG2-S-G2 is distinctly higher than that of HepG2-neo-F4. B: The CYP4F3 protein expression of HepG2-S-G2 is distinctly lower than that of HepG2-neo-F4. β-actin: internal reference protein. The neo-F4 and S-G2 marked on the top of figure A and B respectively represent HepG2-neo-F4 and HepG2-S-G2 cells. The expression of OXCT1 and CYP4F3 in two cells were detected by western blot. 图 6 HepG2-S-G2细胞与HepG2-neo-F4细胞中OXCT1、CYP4F3蛋白表达差异 Fig. 6 The differential protein expression of OXCT1 and CYP4F3 in the HepG2-S-G2 and HepG2-neo-F4 cells |
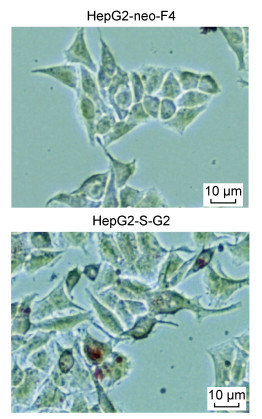
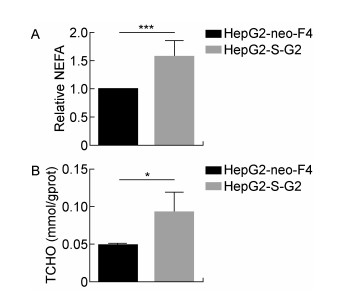
为了验证HBsAg对细胞脂质合成、积累的表型具有促进作用,通过油红O染色和检测细胞NEFA、TCHO水平来评估HepG2-S-G2细胞、HepG2-neo-F4细胞的脂质水平。结果显示,与HepG2-neo-F4细胞相比,HepG2-S-G2细胞中出现更加明显的脂质堆积,形成更明显脂滴,如图 7。另外,HepG2-S-G2细胞中NEFA的相对增长倍数、TCHO的浓度水平均显著高于对照细胞,结果如图 8所示。
|
| The lipid droplets were stained with the cell oil red O staining kit and observed in the bright field of the ordinary inverted microscope. The red signal shows the lipid accumulation, and the scale is 10 μm. 图 7 细胞脂滴的油红O染色 Fig. 7 Oil red O staining of cellular lipid droplets |
|
| A: Taking HepG2-neo-F4 as the control, Y-axis shows the intracellular NEFA fold change. B: Y-axis shows the intracellular TCHO concentration. * 0.01 < P < 0.05; *** 0.000 1 < P < 0.001. 图 8 HepG2-S-G2细胞与HepG2-neo-F4细胞的脂质水平 Fig. 8 The lipid level of HepG2-S-G2 and HepG2-neo-F4 cells |
上述结果表明,HBsAg上调细胞脂质代谢,促进脂质合成和积累。为研究下调脂质合成是否能有效降低HBsAg水平,即两者之间是否存在相互调控机制,选用辛伐他汀和洛伐他汀对稳定转染HBV复制子的HepG2.2.15细胞进行降脂处理。结果表明,降脂处理在该细胞系中表现出显著减少HBsAg分泌的作用,与对照组相比,1 μmol/L、5 μmol/L的辛伐他汀和洛伐他汀处理组的细胞外HBsAg水平分别呈剂量依赖性降低(图 9)。

|
| In order to eliminate the influence of cell proliferation, the average HBsAg secretion level of each cell was calculated by the ratio of HBsAg compared with that of DMSO group after normalized by CCK8 in Y-axis. The percentage of HBsAg level in each group was shown, and the inhibition rate of HBsAg in each group was obtained by subtracting this percentage from 100%. *0.01 < P < 0.05, **0.001 < P < 0.01, ****P < 0.000 1. 图 9 他汀类药物对HepG2.2.15细胞分泌HBsAg的抑制作用 Fig. 9 The inhibitory effect of statins on HBsAg secretion by the HepG2.2.15 cells |
HBV是包膜病毒,大量HBsAg的合成与分泌可干扰宿主细胞正常生理活动,尤其是脂质代谢等途径。本研究发现,HBsAg通过调控关键酶基因的转录与蛋白表达进而影响细胞脂质代谢途径,同时HBsAg上调多种脂肪酸合成通路、抑制胆固醇消耗途径。其中HBsAg促进柠檬酸循环的结果与已报道的HBV pre-S2通过激活三磷酸腺苷(adenosine triphosphate,ATP)柠檬酸盐裂解酶促进糖酵解和脂质生物合成机制[20]存在相似之处。这些结果提示HBsAg可通过不同通路引起脂质代谢上调,其具体机制有待进一步探究。
脂滴是细胞的一种动态的能量储存结构,主要包含中性脂质。当发生代谢紊乱或能量过剩时,往往导致肝脏脂滴中异常的脂质堆积。本研究通过油红O染色发现肝细胞中形成大而明显脂滴,提示持续表达HBsAg的肝细胞发生脂质代谢紊乱。目前,通常认为脂滴形成于内质网。研究表明,内质网应激会导致肝细胞脂滴积累[21]。因此,提示HBsAg引起的脂滴中脂质堆积可能存在内质网应激相关机制。另外,在脂质代谢失衡引发的肝纤维化、HCC等多种肝脏疾病中,也都伴随着血清脂质水平的显著变化。研究表明,与CHB患者相比,HBV相关的肝硬化、HCC患者血清TCHO和甘油三酯水平均显著降低。而与HBV相关肝硬化患者相比,HBV相关HCC患者血清中TCHO水平显著升高[22]。本研究利用酶法检测细胞脂质水平的结果显示,与对照细胞相比,HepG2-S-G2细胞内TCHO、NEFA水平均显著升高,表明HBsAg引起的基因转录水平差异,进一步造成相应蛋白水平变化并引发细胞调控导致实质性的脂质合成上调。同时该表型也与分析总结的HBsAg引起关键酶的差异表达来调控脂质代谢结果一致。总的来看,脂代谢差异基因编码的关键酶主要参与脂肪酸和胆固醇的代谢过程,起到增加合成、减少消耗的作用,推测细胞通过以上相关代谢的变化来维持HBsAg持续性表达与分泌的物质、能量需要。
脂质代谢的上调可以促进HBx表达,两者存在相互调控机制。研究表明,HBx可增强甾醇反应元件结合蛋白1(sterol regulatory element-binding protein 1,SREBP1)和过氧化物酶体增殖物激活受体γ的转录活性及肝脂肪酸结合蛋白1的表达,导致肝脏脂质积累[9, 10];而脂肪酸积累通过活性氧和Ca2+信号维持HBx稳定并促进肝脏炎症基因表达[11]。为了探讨脂质代谢与HBsAg是否存在相互调控作用,本研究利用辛伐他汀和洛伐他汀对稳定转染HBV复制子的HepG2.2.15细胞进行处理,发现降脂能够显著降低HBsAg水平,提示脂质代谢可对HBsAg存在调控作用,降脂可能成为降低HBsAg的有效途径。他汀类药物通常被作为3-羟基-3-甲基戊二酸单酰辅酶A(3-hydroxy-3-methylglutaryl coenzyme A,HMG-CoA)还原酶抑制剂,靶向抑制肝细胞HMG-CoA转化为胆固醇前体-甲羟戊酸,阻断肝脏中胆固醇的产生。同时,他汀类药物通过促进SREBP移位至细胞核并增强低密度脂蛋白受体基因表达,使得肝脏释放出更多受体来清除低密度脂蛋白胆固醇[23]。曾有报道称,洛伐他汀并不抑制HBsAg的mRNA转录[24]。HBsAg本质上是一种糖蛋白,可利用宿主脂质自行组装成大量22 nm的亚病毒颗粒并分泌到胞外[25]。作为寡糖载体,多萜醇磷酸盐是内质网腔中糖蛋白N连接糖基化的必需辅助因子,而洛伐他汀可显著减少多萜醇合成[26],推测他汀类药物可能干扰HBsAg的合成。另外,胆固醇及其衍生物是亚病毒颗粒的主要成分之一,他汀类药物可能通过降低胆固醇含量对亚病毒颗粒的产生与分泌发挥重要作用[25]。总之,他汀类药物抑制HepG2.2.15细胞HBsAg分泌的机制值得进一步研究。
本研究分析了稳定表达HBsAg的细胞系HepG2-S-G2与对照细胞系HepG2-neo-F4的脂质代谢通路中限速酶基因转录组水平差异,并初步探讨了脂质代谢与HBsAg的相互作用关系,为进一步理解HBV与宿主脂质代谢的相互调控机制提供了参考。
| [1] |
World Health Organization. Hepatitis B[EB/OL]. Geneva: World Health Organization, 2020: (2020-07-27). https://www.who.int/zh/news-room/fact-sheets/detail/hepatitis-b.
|
| [2] |
Gluchowski NL, Becuwe M, Walther TC, Farese RV Jr. Lipid droplets and liver disease: From basic biology to clinical implications[J]. Nat Rev Gastroenterol Hepatol, 2017, 14(6): 343-355.
[DOI]
|
| [3] |
Tu T, Budzinska MA, Shackel NA, Urban S. HBV DNA integration: Molecular mechanisms and clinical implications[J]. Viruses, 2017, 9(4): 75.
[DOI]
|
| [4] |
Li Y, Xia Y, Cheng X, Kleiner DE, Hewitt SM, Sproch J, Li T, Zhuang H, Liang TJ. Hepatitis B surface antigen activates unfolded protein response in forming ground glass hepatocytes of chronic hepatitis B[J]. Viruses, 2019, 11(4): 386.
[DOI]
|
| [5] |
Su IJ, Wang HC, Wu HC, Huang WY. Ground glass hepatocytes contain pre-S mutants and represent preneoplastic lesions in chronic hepatitis B virus infection[J]. Gastroenterol Hepatol, 2008, 23(8 Pt 1): 1169-1174.
[URI]
|
| [6] |
Cheng S, Zhang B, Du JY, Jin YH, Lang HY, Zeng LH. Hepatitis B surface antigen promotes the invasion of hepatitis B virus-related hepatocellular carcinoma cells by upregulation of toll-like receptor 2[J]. Viral Immunol, 2017, 30(3): 232-239.
[DOI]
|
| [7] |
Zhu D, Liu L, Yang D, Fu S, Bian Y, Sun Z, He J, Su L, Zhang L, Peng H, Fu Y. Clearing persistent extracellular antigen of hepatitis B virus: An immunomodulatory strategy to reverse tolerance for an effective therapeutic vaccination[J]. J Immunol, 2016, 196(7): 3079-3087.
[DOI]
|
| [8] |
Satoh O, Umeda M, Imai H, Tunoo H, Inoue K. Lipid composition of hepatitis B virus surface antigen particles and the particle-producing human hepatoma cell lines[J]. J Lipid Res, 1990, 31(7): 1293-1300.
[DOI]
|
| [9] |
Hwang KB, Kyaw YY, Kang HR, Seong MS, Cheong J. Mitochondrial dysfunction stimulates HBV gene expression through lipogenic transcription factor activation[J]. Virus Res, 2020, 277: 197842.
[DOI]
|
| [10] |
Wu YL, Peng XE, Zhu YB, Yan XL, Chen WN, Lin X. Hepatitis B virus X protein induces hepatic steatosis by enhancing the expression of liver fatty acid binding protein[J]. J Virol, 2015, 90(4): 1729-1740.
[URI]
|
| [11] |
Cho HK, Kim SY, Yoo SK, Choi YH, Cheong J. Fatty acids increase hepatitis B virus X protein stabilization and HBx-induced inflammatory gene expression[J]. FEBS J, 2014, 281(9): 2228-2239.
[DOI]
|
| [12] |
Wang Y, Wu T, Hu D, Weng X, Wang X, Chen PJ, Luo X, Wang H, Ning Q. Intracellular hepatitis B virus increases hepatic cholesterol deposition in alcoholic fatty liver via hepatitis B core protein[J]. J Lipid Res, 2018, 59(1): 58-68.
[DOI]
|
| [13] |
Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: A cohort study[J]. Hepatology, 2017, 65(3): 828-835.
[DOI]
|
| [14] |
Chung TH, Kim MC, Kim CS. Association between Hepatitis B surface antigen seropositivity and metabolic syndrome[J]. Korean J Fam Med, 2014, 35(2): 81-89.
[DOI]
|
| [15] |
Sells MA, Chen ML, Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA[J]. Proc Natl Acad Sci U S A, 1987, 84(4): 1005-1009.
[DOI]
|
| [16] |
Tian X, Zhao C, Ren J, Ma ZM, Xie YH, Wen YM. Gene-expression profiles of a hepatitis B small surface antigen-secreting cell line reveal upregulation of lymphoid enhancer-binding factor 1[J]. J Gen Virol, 2007, 88(Pt 11): 2966-2976.
[URI]
|
| [17] |
Lin X, Qian GS, Lu PX, Wu L, Wen YM. Full-length genomic analysis of hepatitis B virus isolates in a patient progressing from hepatitis to hepatocellular carcinoma[J]. J Med Virol, 2001, 64(4): 299-304.
[URI]
|
| [18] |
Huang D, Li T, Wang L, Zhang L, Yan R, Li K, Xing S, Wu G, Hu L, Jia W, Lin SC, Dang CV, Song L, Gao P, Zhang H. Hepatocellular carcinoma redirects to ketolysis for progression under nutrition deprivation stress[J]. Cell Res, 2016, 26(10): 1112-1130.
[DOI]
|
| [19] |
Madec S, Cerec V, Plée-Gautier E, Antoun J, Glaise D, Salaun JP, Guguen-Guillouzo C, Corlul A. CYP4F3B expression is associated with differentiation of HepaRG human hepatocytes and unaffected by fatty acid overload[J]. Drug Metab Dispos, 2011, 39(10): 1987-1996.
[DOI]
|
| [20] |
Teng CF, Wu HC, Hsieh WC, Tsai HW, Su IJ. Activation of ATP citrate lyase by mTOR signal induces disturbed lipid metabolism in hepatitis B virus pre-S2 mutant tumorigenesis[J]. J Virol, 2015, 89(1): 605-614.
[DOI]
|
| [21] |
Lee JS, Zheng Z, Mendez R, Ha SW, Xie Y, Zhang K. Pharmacologic ER stress induces non-alcoholic steatohepatitis in an animal model[J]. Toxicol Lett, 2012, 211(1): 29-38.
[DOI]
|
| [22] |
Wu T, Zheng X, Yang M, Zhao A, Li M, Chen T, Panee J, Jia W, Ji G. Serum lipid alterations identified in chronic hepatitis B, hepatitis B virus-associated cirrhosis and carcinoma patients[J]. Sci Rep, 2017, 7: 42710.
[DOI]
|
| [23] |
Stancu C, Sima A. Statins: mechanism of action and effects[J]. J Cell Mol Med, 2001, 5(4): 378-387.
[DOI]
|
| [24] |
Lin YL, Shiao MS, Mettling C, Chou CK. Cholesterol requirement of hepatitis B surface antigen (HBsAg) secretion[J]. Virology, 2003, 314(1): 253-260.
[DOI]
|
| [25] |
Patzer EJ, Nakamura GR, Yaffe A. Intracellular transport and secretion of hepatitis B surface antigen in mammalian cells[J]. J Virol, 1984, 51(2): 346-353.
[DOI]
|
| [26] |
Carlberg M, Dricu A, Blegen H, Wang M, Hjertman M, Zickert P, Höög A, Larsson O. Mevalonic acid is limiting for N-linked glycosylation and translocation of the insulin-like growth factor-1 receptor to the cell surface: Evidence for a new link between 3-hydroxy-3-methylglutaryl-coenzyme a reductase and cell growth[J]. J Biol Chem, 1996, 271(29): 17453-17462.
[DOI]
|
 2020, Vol. 15
2020, Vol. 15


