2. 上海交通大学附属第六人民医院影像放射科, 上海 200123;
3. 上海交通大学附属第六人民医院脊柱外科, 上海 200123
2. Department of Radiology, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai 200123, China;
3. Spine Surgery, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai 200123, China
化脓性脊柱炎(pyogenic vertebral osteomyelitis,PVO)又称脊柱化脓性骨髓炎,是由细菌感染引起的累及椎体、椎间盘和(或)韧带的疾病[1]。据报道,近年来发达国家PVO的年发病率已从1998年的2.9/100 000上升至2013年的5.4/100 000[2]。该病主要通过血源传播,好发于青壮年,老年人和免疫力低下者也容易发生。近年来,随着人口老龄化加剧,慢性消耗性疾病、脊柱侵袭性操作等增多,其发病率逐步上升[2]。抗生素的应用也使其起病隐匿或呈慢性化,临床容易误诊为脊柱结核、布鲁氏菌所致脊柱感染等。PVO虽病死率不高,但进展迅速,2~4周即可发展为骨质破坏,若不及时治疗,可导致脊柱功能障碍或持续疼痛,严重影响生活质量[3]。对于PVO,临床上一般采取制动、应用抗生素等保守治疗,对脊柱不稳定或保守治疗无效者采取病灶清除、植骨融合及内固定等手术治疗[4]。本研究对上海交通大学附属第六人民医院感染病科2009年1—12月收治的20例葡萄球菌感染所致PVO患者的临床资料进行分析总结,期望为PVO的诊断及进一步规范化治疗提供理论依据。
1 材料与方法 1.1 研究对象选取2009年1—12月上海交通大学附属第六人民医院感染病科收治入院的20例葡萄球菌感染所致PVO患者,其中男性9例(45%),女性11例(55%),年龄15~72岁,平均年龄(52.62±13.52)岁。入选标准:原发性椎体感染,均有椎体感染的影像学证据,以血培养或脓液培养检出葡萄球菌作为确诊证据。排除标准:临床资料大量缺失者、有脊柱手术史伴或不伴植入物者。
1.2 研究方法回顾性分析患者的感染途径、临床特点(症状、体征)、实验室检查指标[(血常规、生化、红细胞沉降率(erythrocyte sedimentation rate,ESR)、C反应蛋白(C-reactive protein,CRP)、降钙素原(procalcitonin,PCT)、铁蛋白]、影像学检查[计算机断层扫描(computed tomography,CT)、磁共振成像(magnetic resonance imaging,MRI)、单光子发射计算机断层扫描(emission computed tomography,ECT)]、病理检查、病原学检查和药敏试验结果等。所有患者均采血进行血培养;对形成椎旁脓肿者行CT引导下置管引流,留取脓液进行培养;对血培养阴性、无脓液又高度怀疑PVO的患者行CT引导下穿刺活检,二代测序明确病原体并进行病理学检查。采用Oswestry功能障碍指数(Oswestry disability index,ODI)对腰椎病变进行评分:轻度功能障碍为0~20%,中度功能障碍为21%~40%,重度功能障碍为41%~60%,严重腰背痛为61%~80%,卧床为81%~100%[4]。
1.3 统计学分析应用SAS8.1进行统计学分析,计量资料以X±S表示。
2 结果 2.1 临床特征20例PVO患者均未发现皮肤破溃、痈等原发灶。13例(65%)有发热,12例(60%)出现椎旁脓肿,2例(10%)患有糖尿病,3例(15%)感染前有明确的脊柱损伤史(均未发现疖、痈、泌尿道等感染灶)。所有患者均有不同程度的腰痛,从出现腰痛到就诊平均时间约为1个月,1例有明确的发热后腰痛。具体情况如表 1所示。
| Item | Number of cases(n=20) n(%) |
| Age | |
| <50 years | 4(20) |
| ≥50 years | 16(80) |
| Temperature | |
| 36.3~<37.2 ℃ | 7(35) |
| 37.2~<39 ℃ | 6(30) |
| ≥39 ℃ | 7(35) |
| History of spinal injury | 3(15) |
| Paravertebral abscess | 12(60) |
| Hypertension | 4(20) |
| Diabetes | 2(10) |
| Backache | 20(100) |
| Staphylococcus classification | |
| Staphylococcus aureus | 18(90) |
| Staphylococcus lugdunensis | 1(5) |
| Staphylococcus capitis | 1(5) |
| ODI score | |
| 0~20% | 8(40) |
| 21%~40% | 11(55) |
| 41%~60% | 1(5) |
| 61%~80% | 0(0) |
| 81%~100% | 0(0) |
患者病变椎体分布情况如图 1所示:17例(85%)病变在腰椎,2例(10%)在胸椎,1例(5%)在颈椎同时累及腰椎。ODI评分[4]显示,20例PVO患者以轻中度功能障碍为主。
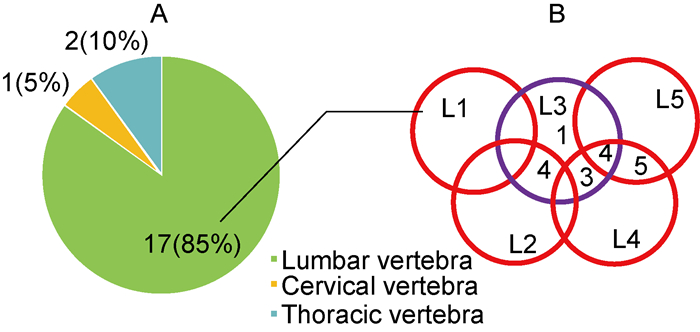
|
| A: Distribution of vertebral infection. B: Distribution of lumbar spine infections. 图 1 PVO患者病变椎体分布 Fig. 1 Distribution of diseased vertebral bodies in patients with PVO |
20例PVO患者血液白细胞计数为6.80×109~12.40×109/L,50%患者升高;CRP、ESR、铁蛋白均升高,分别为60.40~144.00 mg/L、83.50~114.50 mm/h、390.70~928.90 ng/mL;50%患者血小板升高;65%患者血清白蛋白下降,其中4例白蛋白低于30 g/L;PCT基本正常,仅1例轻度升高(表 2)。
| Item | X±S |
| White blood cell count (×109/L) | 10.45±4.82 |
| Neutrophils (%) | 70.40±24.09 |
| Hemoglobin (g/L) | 109.43±45.04 |
| Platelet (×109/L) | 347.5±52.31 |
| CRP (mg/L) | 107.15±67.59 |
| Albumin (g/L) | 37.81±11.61 |
| ESR (mm/h) | 95.50±21.21 |
| Procalcitonin (ng/mL) | 0.31±0.78 |
| Ferritin (ng/mL) | 653.86±351.68 |
CT示病变椎体骨质破坏,伴周围软组织肿胀,增强后示椎旁软组织呈边缘强化,中心为无强化液化坏死区(图 2)。MRI示病变椎体及椎间盘破坏区异常信号灶,呈T1加权成像(T1-weighted imaging,T1WI)低信号、T2WI压脂高信号,增强后可见明显强化,椎旁软组织肿胀及T2WI压脂高信号(见图 3)。ECT示病变椎体不均匀放射性摄取增高(图 4)。图 2A1~A3、图 3A1~A3、图 4A为同一患者的影像学表现,为64岁男性,诊断为耐甲氧西林金黄色葡萄球菌(methicillin-resistant Staphylococcus aureus,MRSA)感染所致PVO(胸椎)。图 2B1~B3、图 3B1~B3、图 4B分别来自3例PVO(腰椎)患者。
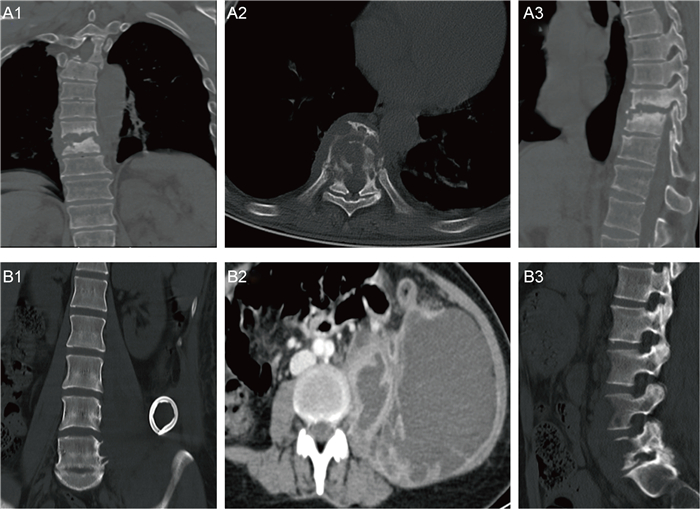
|
| A: Male, 64 years old, T9-T11, MRSA infection. Increased density in T9-T11 vertebral bodies was observed, with bone destruction and abnormal density in surrounding soft tissues. B: Female, 40 years old, L4-L5, methicillin-sensitive Staphylococcus aureus (MSSA) infection. Multiple cystic low-density shadows could be observed on the left psoas major muscle, with uneven internal density. CT value was about 25 HU. After enhancement, the wall was obviously strengthened, but the central liquefied necrosis area was not obviously strengthened. The left cortical bone of the L4 and L5 vertebrae was affected and not smooth. 图 2 PVO的CT表现 Fig. 2 CT image of PVO |
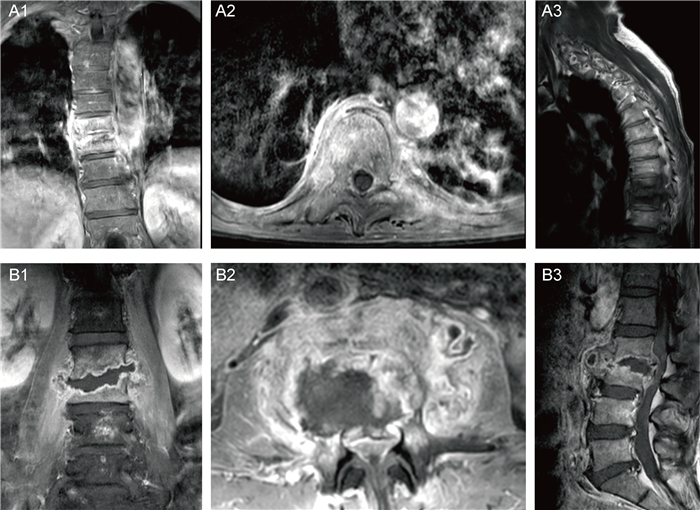
|
| A: Male, 64 years old, T9-T11, MRSA infection. The signals in T9-T11 intervertebral disc and vertebral body were abnormal, with low signal on T1WI, high signal on fat-suppression T2WI, and swelling of the surrounding soft tissues. B: Male, 59 years old, L2-L3, MSSA infection. The lumbar spine was smoothed and the physiological curve became straight. L2-L3 intervertebral space stenosis with bone destruction in the vertebral body was observed, accompanied by swelling of the surrounding soft tissues. After enhancement, a ring-shaped enhancement was observed locally, with corresponding spinal canal stenosis, and the dural sac was obviously compressed. 图 3 PVO的MRI表现 Fig. 3 MRI image of PVO |
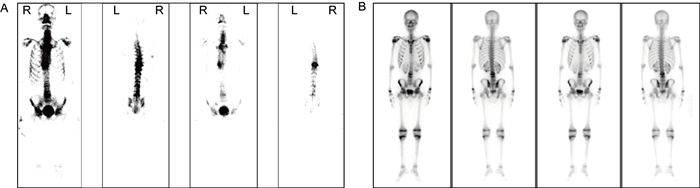
|
| A: Male, 64 years old, T9-T11, MRSA infection. Radioactive uptake of T9-T10 vertebral bodies increased. B: Male, 15 years old, L4-L5, MRSA infection. Local radioactive uptake at the left edge of L4-L5 vertebrae slightly increased. 图 4 PVO的ECT表现 Fig. 4 ECT image of PVO |
20例PVO患者中,11例血培养阳性,12例脓液培养阳性,其中6例培养双阳性。对血培养阴性、无脓液又高度怀疑PVO的3例患者行CT引导下腰椎病变穿刺活检并使用二代测序方法明确病原体。共检出金黄色葡萄球菌18株(90%),路邓葡萄球菌1株(5%),头状葡萄球菌1株(5%)。
椎体穿刺活检病理切片示炎性细胞浸润伴纤维素样坏死和炎性肉芽组织增生(图 5)。
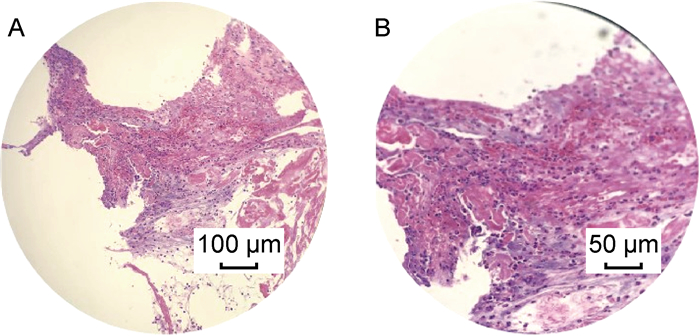
|
| Female, 40 years old, L3-L4, MSSA infection. A small amount of nucleus pulposus fibrocartilage tissue with large fibrinous necrosis, local purulent inflammatory exudation, and local inflammatory granulation tissue hyperplasia were observed in L3-L4 intervertebral space. 图 5 PVO的病理学表现 Fig. 5 Pathological manifestations of PVO |
对从患者体内分离培养得到的细菌进行药敏试验。18株金黄色葡萄球菌中,对青霉素耐药14株(77.8%),对氨苄西林/舒巴坦和头孢曲松耐药6株(33.3%),对左氧氟沙星耐药8株(44.4%),对克林霉素耐药9株(50%),对磺胺甲唑+甲氧苄啶(复方新诺明)耐药3株(16.7%),对利福平耐药2株(11.1%),对万古霉素均敏感(表 3)。18株金黄色葡萄球菌中检出MRSA 4株。头状葡萄球菌对克林霉素、红霉素、环丙沙星、左氧氟沙星、莫西沙星、苯唑西林、青霉素耐药。邓路葡萄球菌对阿莫西林/克拉维酸、氨苄西林、环丙沙星、左氧氟沙星、莫西沙星、头孢曲松、苯唑西林、青霉素、氨苄西林/舒巴坦耐药。
| Antibiotics | Resistant(n) | Sensitive(n) |
| Penicillin | 14 | 4 |
| Ampicillin/sulbactam | 6 | 12 |
| Ceftriaxone | 6 | 12 |
| Levofloxacin | 8 | 10 |
| Clindamycin | 9 | 9 |
| Methicillin | 4 | 14 |
| Compound sulfamethoxazole | 3 | 15 |
| Rifampin | 2 | 16 |
| Vancomycin | 0 | 18 |
所有患者严格卧床制动。12例椎旁脓肿患者在CT引导下行穿刺引流术加静脉滴注敏感抗生素治疗6周,体温控制在正常范围,白细胞计数、ESR、CRP恢复正常或接近正常,引流管不再引出脓液48~72 h后拔除;8例采用静脉滴注敏感抗生素保守治疗,临床体征及实验室检查指标恢复正常后出院,改为口服抗生素6周。所有患者恢复及预后良好。
3 讨论本研究发现PVO好发于腰椎(占60%),其中L3、L4最易发生,L1、L2较少见,病变常累及相邻2个椎体;较少发生于胸椎(占30%),仅10%发生于颈椎,与先前文献报道一致[5]。成人椎体由内小动脉供血,血流缓慢,易受细菌侵袭导致血管闭塞坏死、骨梗死及椎体骨髓炎或脊柱炎[6]。随着骨质的破坏,感染扩散到邻近组织,形成椎间盘炎,使椎间盘和两个相邻椎体受累[7]。
本研究中20例PVO患者均有不同程度的腰痛,65%伴发热。有文献显示,PVO的主要临床症状为腰背痛(占86%),剧烈疼痛常提示硬膜外脓肿形成,其次是发热(占60%)[8]。白细胞计数、中性粒细胞百分比、ESR、CRP、PCT等在诊断感染性疾病时有重要价值,ESR和CRP可用于疗效评估,PCT对脊柱感染的敏感性不如CRP[9]。脊柱感染的危险因素主要包括高龄、营养不良、免疫抑制、糖尿病、静脉使用药物、肾衰竭、败血症、脊柱手术、血管内置管、体内异物等[10],但本研究中糖尿病患者比例不高,需增大样本量进一步探讨。PVO与结核性脊柱炎的症状和体征有较高的相似性,很难区分两者。
近年来由金黄色葡萄球菌引起的PVO病例逐年增加[11]。在西方国家,金黄色葡萄球菌感染所致PVO占60%[12]。抗生素的滥用导致多重耐药菌不断出现,因此了解细菌耐药特点对尚未取得培养结果的患者经验性应用抗生素十分重要。本研究中金黄色葡萄球菌对抗生素的耐药率由高到低依次为青霉素、克林霉素、左氧氟沙星、头孢曲松和氨苄西林。但本研究可能高估了导致PVO的细菌对喹诺酮的耐药率,有数据表明利福平+左氧氟沙星能有效治疗78%的PVO患者[13-14]。目前,MRSA耐药问题严重且致病呈多样性,导致临床上抗生素选择受限,而如果早期感染得不到有效控制,极可能导致严重后果,如并发神经功能损害、中毒性休克等,因此早期识别MRSA感染对控制PVO尤为重要[15]。头状葡萄球菌和邓路葡萄球菌为凝固酶阴性条件致病菌,本研究结果显示这两种细菌为多重耐药菌,且药敏试验结果与金黄色葡萄球菌相似。现有研究也表明,凝固酶阴性葡萄球菌(如表皮葡萄球菌、邓路葡萄球菌、头状葡萄球菌等)正逐渐成为多重耐药病原体[16-17],不能忽视其导致的疾病。
化脓性细菌(最常见的是金黄色葡萄球菌、肠杆菌等)可通过蛋白水解酶来消化椎间盘和端板,容易导致骨髓炎,且病情进展迅速,形成腰大肌脓肿。非化脓性脊柱炎多由结核分枝杆菌引起,不产生蛋白水解酶,因此常进展缓慢,可导致硬膜外和椎旁组织受累、软组织钙化[11]。脊柱MRI检查在反映软组织损伤方面具有优势,可在一定程度上识别脊柱结核(椎体溶骨性骨质破坏,T2WI呈高信号或高低混杂信号,部分病例可见硬化边,增强扫描骨内脓肿多呈花环状、轮辐状强化)[16],用于早期诊断及随访。CT对描述骨骼和评估骨骼破坏很敏感,增强时出现典型强化,可用于病程长的患者。对于使用上述常用影像学检查仍不能区分化脓性和非化脓性脊柱炎者,可考虑行全身骨扫描检查。CT引导下穿刺活检进行病原学诊断具有很高的特异性[4],是PVO诊断的金标准,还可在未获得血培养结果又高度怀疑PVO时提供诊断依据。但穿刺活检为有创性检查,不是所有患者都能接受,故本研究仅对3例血培养阴性且无脓液的患者进行了CT引导下穿刺活检并二代测序以明确病原体。
对于PVO的治疗方式有不同观点。大多数学者认为,静脉滴注抗生素4~6周可使临床体征及实验室指标恢复正常,后续再口服抗生素6周可达到治疗目的[18-20]。也有学者认为,抗生素只在早期炎症未波及椎间盘时有效,对于病程超过2周、中毒症状明显、椎管硬膜外脓肿形成者须及早进行手术治疗。本研究中20例PVO患者均采用抗生素保守治疗,对形成腰大肌脓肿者置管引流,无脓液继续排出48~72 h后拔管,患者痊愈且预后良好。因此,大多数葡萄球菌感染所致PVO经保守治疗达到痊愈是可行的。对于缺乏药敏试验结果者,可首先使用广谱抗生素覆盖常见病原体,高剂量左氧氟沙星联合利福平可有效治疗PVO[21]。本研究对MRSA感染者进行了定期随访,以MRI及相关实验室检查(白细胞计数、中性粒细胞百分比、ESR、CRP)作为随访指标。结果显示,在疾病早期这些指标均明显升高。对1例以腰痛、发热为主要症状的15岁男孩进行随访:其入院时CRP、ESR及白细胞计数均升高,影像学检查提示腰椎感染,血培养为MRSA;经敏感抗生素治疗后,患者白细胞计数于2周后恢复正常,CRP和ESR于2个月后恢复正常,PCT一直处于正常水平,MRI虽显示椎体水肿及腰软组织肿胀逐渐好转,但在3个月时仍能观察到椎骨病变。虽然该病例的炎症指标先于影像学恢复正常,但如何合理选择疗程仍须加大样本量进一步探讨。
综上所述,本研究发现金黄色葡萄球菌是葡萄球菌感染所致PVO的主要致病菌,CT和MRI是诊断脊柱感染的有效辅助检查。静脉滴注敏感抗生素6周外加口服抗生素6周,同时对形成椎旁脓肿者行置管引流可达到治愈效果。在疾病恢复期,血清学指标可能先于影像学表现恢复正常,但两者均能作为疾病诊断及预后的重要指标。本研究纳入的病例数较少,结论存在一定的局限性,须在更大样本队列中进行验证。
| [1] |
Tali ET. Spinal infections[J]. Eur J Radiol, 2004, 50(2): 120-133.
[DOI]
|
| [2] |
Yu D, Kim SW, Jeon I. Antimicrobial therapy and assessing therapeutic response in culture-negative pyogenic vertebral osteomyelitis: a retrospective comparative study with culture-positive pyogenic vertebral osteomyelitis[J]. BMC Infect Dis, 2020, 20(1): 939.
[DOI]
|
| [3] |
张霞, 王军峰, 张德增, 唐孝春, 陈纪金, 孟庆学. 脊柱化脓性感染的影像学诊断与鉴别诊断[J]. 医学影像学杂志, 2020, 30(8): 1539-1542. [CNKI]
|
| [4] |
Wang K, Zhang C, Cheng C, Jian F, Wu H. Radiographic and clinical outcomes following combined oblique lumbar interbody fusion and lateral instrumentation for the treatment of degenerative spine deformity: a preliminary retrospective study[J]. Biomed Res Int, 2019, 2019: 5672162.
[DOI]
|
| [5] |
Cottle L, Riordan T. Infectious spondylodiscitis[J]. J Infect, 2008, 56(6): 401-412.
[DOI]
|
| [6] |
Govender S. Spinal infections[J]. J Bone Joint Surg Br, 2005, 87(11): 1454-1458.
|
| [7] |
Shenoy K, Singla A, Krystal JD, Razi AE, Kim YH, Sharan AD. Discitis in adults[J]. JBJS Rev, 2018, 6(6): e6.
[DOI]
|
| [8] |
陈秋洪, 陈雷, 李杰. 化脓性脊柱炎诊疗进展[J]. 福建医科大学学报, 2020, 54(1): 62-66. [CNKI]
|
| [9] |
陈婉婷, 刘泳坚, 严彩英, 赵晓梅, 周守国, 张家雄, 衣利磊. 结核性脊柱炎与化脓性脊柱炎MR表现及鉴别诊断[J]. 国际感染病学(电子版), 2019, 8(3): 201. [CNKI]
|
| [10] |
郑月焕, 曹鹏, 陈哲, 周泽著. 脊柱感染[J]. 国际骨科学杂志, 2014, 35(1): 24-26. [CNKI]
|
| [11] |
Babic M, Simpfendorfer CS. Infections of the spine[J]. Infect Dis Clin North Am, 2017, 31(2): 279-297.
[DOI]
|
| [12] |
Tali ET. Spinal infection[J]. Neuroimaging Clin N Am, 2015, 25(2): xv.
[DOI]
|
| [13] |
Bernard L, Dinh A, Ghout I, Simo D, Zeller V, Issartel B, Le Moing V, Belmatoug N, Lesprit P, Bru JP, Therby A, Bouhour D, Dénes E, Debard A, Chirouze C, Fèvre K, Dupon M, Aegerter P, Mulleman D; Duration of Treatment for Spondylodiscitis (DTS) study group. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial[J]. Lancet, 2015, 385(9971): 875-882.
[DOI]
|
| [14] |
Luzzati R, Giacomazzi D. The empirical antibiotic therapy of pyogenic vertebral osteomyelitis[J]. Semin Arthritis Rheum, 2012, 41(4): e9.
[DOI]
|
| [15] |
Otto M. Community-associated MRSA: what makes them special[J]. Int J Med Microbiol, 2013, 303(6-7): 324-330.
[DOI]
|
| [16] |
Babu E, Oropello J. Staphylococcus lugdunensis: the coagulase-negative staphylococcus you don't want to ignore[J]. Expert Rev Anti Infect Ther, 2011, 9(10): 901-907.
[DOI]
|
| [17] |
冯洁仪, 邓述欢, 赖少芬, 黎艳枝. 头状葡萄球菌在临床感染的分布状况和耐药分析[J]. 检验医学与临床, 2019, 16(1): 109-111. [CNKI]
|
| [18] |
Jeong DK, Lee HW, Kwon YM. Clinical value of procalcitonin in patients with spinal infection[J]. J Korean Neurosurg Soc, 2015, 58(3): 271-275.
[DOI]
|
| [19] |
Chen WC, Wang JL, Wang JT, Chen YC, Chang SC. Spinal epidural abscess due to Staphylococcus aureus: clinical manifestations and outcomes[J]. J Microbiol Immunol Infect, 2008, 41(3): 215-221.
[URI]
|
| [20] |
Seyman D, Berk H, Sepın-Ozen N, Kızılates F, Turk CC, Buyuktuna SA, Inan D. Successful use of tigecycline for treatment of culture-negative pyogenic vertebral osteomyelitis[J]. Infect Dis (Lond), 2015, 47(11): 783-788.
[DOI]
|
| [21] |
Deng W, Long Q, Zeng J, Li P, Yang W, Chen X, Xie J. Mycobacterium tuberculosis PE_PGRS41 enhances the intracellular survival of M. smegmatis within macrophages via blocking innate immunity and inhibition of host defense[J]. Sci Rep, 2017, 7: 46716.
[DOI]
|
 2021, Vol. 16
2021, Vol. 16



