2. 海军军医大学教务处,上海 200433;
3. 西藏军区总医院检验科,西藏自治区 拉萨 850007
2. Office of Academic Affairs, Naval Military Medical University, Shanghai 200433, China;
3. Department of Clinic Laboratory Diagnostics, General Hospital of Tibet Military Area Command of PLA, Lhasa 850007, Tibet, China
肠道病毒A71型(enterovirus A71,EV-A71)属于小RNA病毒科肠道病毒属人肠道A组病毒,是导致手足口病(hand-foot-mouth disease,HFMD)的主要病原体之一。HFMD主要发病人群为5岁以下婴幼儿,临床表现为发热,手、足、臀部以及口腔黏膜等部位出现疱疹等症状;少数患儿可发展为重症,出现中枢神经系统(central nervous system,CNS)病变,包括无菌性脑膜炎、脑干脑炎、脑脊髓炎以及神经源性肺水肿等,严重威胁患儿的生命健康[1]。HFMD特别是重症病例多由EV-A71感染所致,目前尚无特异高效的抗病毒药物,临床上仍以对症治疗为主[2]。
EV-A71主要经粪-口途径传播,首先在宿主肠道中复制,随后入血引发肠道外感染,但其侵入肠道细胞的机制尚不清楚[3-4]。病毒在感染宿主时,必须利用宿主细胞的各种分子及相关信号通路才能完成自身的生命周期,其中细胞膜转运相关分子对病毒的入侵、复制以及释放有感染性的子代病毒颗粒具有重要意义,已成为抗病毒药物筛选的重要靶标[5-6]。
宿主细胞转运相关膜蛋白在宿主细胞的跨膜物质运输、囊泡内吞与分泌中发挥着重要作用,但在感染过程中可能易被病毒“劫持”并利用,从而参与病毒的入侵、复制和转运。宿主细胞转运相关膜蛋白主要包括ABCC3[7]、去整合素金属蛋白酶10(a disintegrin and metalloprotease 10,ADAM10)[8]、ADP核糖基化因子6(ADP-ribosylation factor 6,ARF6)[9]、小窝蛋白1(caveolin-1,CAV1)[10-11]、发育和分化增强因子2(development and differentiation-enhancing factor 2,DDEF2)[12]、酪氨酸蛋白激酶FYN(protein tyrosine kinase,PTK FYN)[13]、高尔基体重组堆叠蛋白(Golgi reassembly-stacking protein 1,GORASP1)1[14]、Huntingtin相互作用蛋白1相关蛋白(Huntingtin-interacting protein 1-related protein,HIP1R)[15]、intersectin-1(ITSN1)[16]、SLC7A7 [17]、囊泡相关膜蛋白1(vesicle-associated membrane protein 1,VAMP1)[18]、VAMP2[19]、VAMP相关蛋白A(vesicle-associated membrane protein-associated protein A,VAPA)[20]和绒毛蛋白2(villin 2,VIL2)等[21]。人结肠癌细胞系Caco-2在形态学和生理学方面与人小肠黏膜上皮细胞相似。培养成熟的Caco-2为致密单层细胞,具有与正常小肠黏膜上皮细胞相同的极性、紧密连接及微绒毛结构[22]。本研究采用RNA干扰技术,观察上述14个细胞转运相关膜蛋白对EV-A71感染Caco-2细胞的影响,以期为EV-A71感染的预防和治疗提供数据。
1 材料与方法 1.1 材料 1.1.1 试剂与耗材Caco-2细胞购自美国ATCC公司,DMEM培养基、10%胎牛血清购自美国Thermo Fisher Scientific公司,DharmaFECTTM转染试剂购自美国Horizon公司,RNAiso Plus、反转录试剂盒、TB Green Premix Ex Taq试剂盒、RNAiso Plus购自日本TaKaRa公司,CCK-8细胞计数试剂购自日本同仁化学研究所,EV-A71 VP1特异性鼠源单抗10F0购自英国Abcam公司。
1.1.2 引物设计检索美国国家生物技术信息中心(National Center for Biotechnology Information,NCBI)GenBank数据库,获得上述14个转运相关膜蛋白基因的全序列和mRNA序列,利用GeneCard和proteinatlas数据库进行生物学分析,选择编码区作为siRNA设计的靶序列。参照siRNA设计原则[23],并利用GenBank数据库的Blast功能与人类基因组序列进行比对,以确保无同源性;排除antisense链5 ′端连续8个碱基与其他基因配对的潜在siRNA;排除任何一段连续14个碱基与其他基因配对的潜在siRNA。利用RNAi Designer (Invitrogen)在线软件设计进行预评估测定,选择3个最佳动力学参数靶点进入后续实验流程,每一基因共合成3条干扰序列(见表 1)。单链siRNA的合成与纯化由美国Invitrogen公司完成。
| Group | Gene ID | SEQ ID NO. | siRNA target sequence(5′-3′) | RQ (mean±SD)(%) |
| CTRLa | 无 | 100 | ||
| NTb | UUCUCCGAACGUGUCACGUUU | 100±5.28 | ||
| ABCC3c | 8 714 | 1 | CGCUGAUCUUACAACACUAd | 12.08±7.18 |
| 2 | GCUGAUCUUACAACACUAU | 25.55±3.08 | ||
| 3 | UAGUGUUGUAAGAUCAGCG | 21.17±5.44 | ||
| ADAM10c | 102 | 4 | GCUAAUGGCUGGAUUUAUU | 28.19±2.40 |
| 5 | CCCAAAGUCUCUCACAUUAd | 17.64±1.54 | ||
| 6 | GCAAGGGAAGGAAUAUGUA | 43.34±7.24 | ||
| ARF6c | 382 | 7 | CCUCUAACUACAAAUCUUA | 26.51±4.83 |
| 8 | GGAAGGUGCUAUCCAAAAUd | 8.38±4.12 | ||
| 9 | GGAUACAACUAAAGUACGA | 22.41±3.19 | ||
| CAV1c | 857 | 10 | CUAAACACCUCAACGAUGAd | 8.77±2.59 |
| 11 | GCAGUUGUACCAUGCAUUA | 19.99±5.86 | ||
| 12 | GCAAAUACGUAGACUCGGA | 36.59±9.34 | ||
| DDEF2c | 8 853 | 13 | GAAAUCCGCAUUGCAGGUU | 28.34±6.52 |
| 14 | CGAGAUAGCAAACGAGUCA | 33.07±6.61 | ||
| 15 | GGUCCUCUGUCCAAUGCUAd | 16.92±6.15 | ||
| FYNc | 2 534 | 16 | GCUCUGAAAUUACCAAAUCd | 18.40±3.08 |
| 17 | CGCAUGAAUUAUAUCCAUA | 41.92±11.33 | ||
| 18 | CUGUGAAGCAUUCGAGACA | 29.34±7.79 | ||
| GORASP1c | 64 689 | 19 | CUGGAGGUGUUCAAUAUGA | 30.08±9.05 |
| 20 | GAGGACUUCUUUACGCUCAd | 9.35±1.51 | ||
| 21 | GAUCUCUACCACAGAAUAA | 21.47±4.37 | ||
| HIP1Rc | 9 026 | 22 | CAGCUCAACUCGUGAACUAd | 17.54±3.91 |
| 23 | CUGUGGAGAUGUUUGAUUA | 28.18±6.00 | ||
| 24 | CCUCUUCGAUCAGACGUUU | 26.34±2.37 | ||
| ITSN1c | 6 453 | 25 | GGACAUAGUUGUACUGAAAd | 20.79±5.18 |
| 26 | GAUAUCAGAUGUCGAUUGA | 36.52±5.14 | ||
| 27 | GGCCAUAACUGUAGAGGAA | 24.43±6.81 | ||
| SLC7A7c | 9 056 | 28 | CUCUUAACCUUCAUUAACU | 23.97±6.64 |
| 29 | GGCACCACCAUUAAGAAAUd | 11.56±4.13 | ||
| 30 | AGUUAAUGAAGGUUAAGAG | 44.08±6.72 | ||
| VAMP1c | 6 843 | 31 | CUCCUAACAUGACCAGUAAd | 3.82±1.77 |
| 32 | CAUCACAAUUUGAGAGCAG | 24.86±7.49 | ||
| 33 | CCAUCAUCGUGGUAGUUAU | 32.00±5.37 | ||
| VAMP2c | 6 844 | 34 | UAGUUUACUUCAGCUCUUAd | 9.13±2.41 |
| 35 | GCGCAAAUACUGGUGGAAA | 19.42±7.75 | ||
| 36 | CCUCCAAACCUCACCAGUA | 33.53±7.10 | ||
| VAPAc | 9 218 | 37 | CCACAGACCUCAAAUUCAA | 29.48±3.96 |
| 38 | GGCAAAACCUGAUGAAUUA | 49.91±4.17 | ||
| 39 | CCUGAGAGAUGAAGGUUUAd | 15.48±5.59 | ||
| VIL2c | 7 430 | 40 | GCUCAAAGAUAAUGCUAUGd | 12.93±5.29 |
| 41 | GGCAACAGCUGGAAACAGA | 32.28±6.80 | ||
| 42 | CAAGAAGGCACCUGACUUU | 20.34±8.21 | ||
| 注:a:不转染任何siRNA的Caco-2细胞组(空转对照组);b:转染non-targeting siRNA的Caco-2细胞组(阴性对照组);c:转染针对各目的基因siRNA的Caco-2细胞组(实验组);d:实验组在后续实验中采用的siRNA序列。 | ||||
分为不转染任何siRNA的Caco-2细胞组(空转对照组)、转染non-targeting siRNA的Caco-2细胞组(阴性对照组)、转染针对目的基因siRNA的Caco-2细胞组(实验组)。
1.2.2 RNA转染转染步骤参照DharmaFECTTM说明书,将Caco-2细胞以3×104/孔接种于24孔培养板,37 ℃培养,待细胞密度达70%左右进行siRNA转染。取4 μL DharmaFECTTM加至46 μL opti-MEM减血清培养基中,轻柔混匀,室温孵育5 min;另取5 μL siRNA(5 μmol/L)与46 μL opti-MEM混合。孵育结束后,将稀释的DharmaFECT-1转染试剂加入稀释的siRNA中,轻柔吹吸混匀,室温孵育15 min后加至Caco-2细胞中,补加400 μL opti-MEM,使siRNA终浓度为50 nmol/L。6~8 h后更换为含10%胎牛血清的DMEM培养基。
1.2.3 实时荧光定量聚合酶链反应检测各目的基因mRNA水平转染后48 h,去除培养上清液,在细胞中加入1 mL RNAiso Plus裂解液,充分混合,于冰上裂解5~10 min。将混合物转移到EP管,加入1/5体积的氯仿,剧烈振荡15 s,4 ℃ 12 000 r/min离心15 min。将上层水相转移到新的EP管中,加入等体积异丙醇,充分混合,室温静置10 min,于4 ℃ 12 000 r/min离心10 min。弃上清液,加入1 mL冰冷的75%乙醇,4 ℃ 12 000 r/min离心10 min。充分去除上清液,于室温晾干RNA沉淀物,加入去RNA酶水(RNase-free ddH2O)溶解沉淀,得到总RNA。利用反转录试剂盒制备总cDNA,在PCR管中加入5×PrimeScript RT Master Mix 2 μL、total RNA 500 ng,补加RNase-free ddH2O至10 μL。轻柔混匀,于37 ℃反应15 min(反转录),然后于85 ℃灭活5 s。利用TB Green Premix Ex Taq试剂盒进行实时荧光定量聚合酶链反应(real-time polymerase chain reaction,real-time PCR)检测目的基因mRNA水平,反应体系含SYBR Premix Ex Taq 10 μL、forward primer(10 μ mol/L)0.8 μL、reverse primer(10 μ mol/L)0.8 μL、DNA模板2 μL、ddH2O 6.4 μL,总体积20 μL。利用Applied Biosystems 7300Plus仪器(美国)进行两步法扩增,第1步为95 ℃预变性30 s,第2步为95 ℃ 5 s,60 ℃ 30 s,进行40个循环。采用ΔΔCt法计算各靶基因相对表达量。相对定量(relative quantification,RQ)通过如下公式计算:RQ=2-ΔΔCt,其中,ΔΔCt=(Ct处理样本目的基因-Ct处理样本内参基因)-(Ct对照样本目的基因-Ct对照样本内参基因),干扰效率=1-RQ。
1.2.4 细胞毒性实验采用CCK-8法检测转染siRNA对细胞增殖的影响。收集对数生长期细胞,以3 000个/孔的密度接种于96孔板。待细胞融合度约70%时进行siRNA转染,培养48 h,弃去原有培养基,每孔加入含10 μL CCK-8的新鲜培养基100 μL,培养3~4 h后用BioTek多功能酶标仪(美国)在450 nm处检测各孔吸光度。实验独立重复3次,计算平均值,并将结果标准化到空转对照组。
1.2.5 免疫荧光染色法检测EV-A71抗原表达Caco-2细胞转染siRNA 48 h后进行EV-A71感染实验。将培养上清液吸出,用预温的磷酸盐缓冲液(phosphate buffered saline,PBS)洗2次,接种EV-A71(感染复数MOI=0.1),37 ℃孵育2 h后弃去病毒液,用预温的PBS洗3次,加入新鲜培养基继续在37 ℃、5%CO2条件下培养48 h。采用免疫荧光法检测病毒抗原的表达:移去96孔板中的培养液,加入PBS洗2次,每孔加入100 μL预冷的甲醇,置于-20 ℃环境中20 min;用预冷的PBS洗细胞3次,每孔加入100 μL 3%牛血清白蛋白(bovine serum albumin,BSA),室温封闭1 h;每孔加入EV-A71特异性鼠源单抗10F0(1∶1 000稀释)100 μL,4 ℃摇床孵育过夜;用预冷的PBS洗3次,每孔加入AF488荧光标记抗鼠IgG(1∶1 000稀释)100 μL,室温避光孵育1 h,用预冷的PBS避光洗3次;每孔加入细胞核荧光染料4 ′, 6-二脒基-2-苯基吲哚(4 ′, 6-diamidino-2-phenylindole,DAPI)(1∶10 000,PBS稀释),室温避光孵育15 min;用预冷的PBS避光洗3次,于荧光显微镜下检测并计算绿色AF488阳性细胞的比率。
1.2.6 real-time PCR检测细胞中EV-A71载量Caco-2细胞感染病毒后继续培养48 h,用TRIzol提取细胞总RNA,反转录获得cDNA,用real-time PCR检测EV-A71载量。
1.2.7 蛋白质印迹法检测EV-A71蛋白水平细胞培养、转染过程同上。用RIPA蛋白裂解液提取Caco-2细胞总蛋白,定量后取20 μ g蛋白进行10%二聚酸钠-聚丙烯酰胺凝胶电泳(sodium diacetate-polyacrylamide gel electrophoresis,SDA-PAGE),截取相应条带,电转至聚偏二氟乙烯膜(polyvinylidene fluoride,PVDF)。用5%脱脂牛奶封闭蛋白的非特异性位点,与稀释的EV-A71 VP1特异性鼠源单抗10F0(1∶1 000稀释)4 ℃孵育过夜,用含吐温20的Tris-HCl缓冲液(Tris-buffered saline with Tween 20,TBST)漂洗3次,与辣根过氧化物酶(horseradish peroxidase,HRP)标记的兔抗鼠IgG(1∶1 000稀释)于室温孵育2 h,用TBST缓冲液洗3次,最后显色并拍照分析。
1.3 统计学方法采用Graphpad 7.0软件对数据进行统计学分析,计量资料以均值±标准差表示,多组计量资料差异采用单因素方差分析法,组间多重比较采用Bonfferoni t检验,P<0.05代表有统计学意义。
2 结果 2.1 有效siRNA的筛选通过检索NCBI GenBank数据库获得细胞转运相关膜蛋白ABCC3、ADAM10、ARF6、CAV1、DDEF2、FYN、GORASP1、HIP1R、ITSN1、SLC7A7、VAMP1、VAMP2、VAPA和VIL2的全序列和mRNA序列,进行生物信息学分析,选择编码区作为siRNA的靶序列从而设计siRNA。采用体外转染方法,将上述14个siRNA分别转染Caco-2细胞,48 h后采用real-time PCR检测各目的基因相对表达量。结果显示,针对每个目的基因所设计的siRNA均能有效抑制相应基因的表达,有效抑制率达50%以上。选取对每个目的基因干扰效果最佳的siRNA(见表 1中上标“d”的序列)进行后续实验。
2.2 目的基因siRNA的干扰效率高且无细胞毒性将上述各有效siRNA转染Caco-2细胞,48 h后收集细胞,采用real-time PCR检测各目的基因相对表达量,同时采用CCK-8法检测siRNA转染对Caco-2细胞的毒性。结果如图 1所示,与对照组相比,转染各siRNA均能明显抑制相应基因的表达水平(P<0.05),其中转染ABCC3 siRNA(SEQ ID NO: 1)和SLC7A7 siRNA(SEQ ID NO: 29)的干扰效率分别高达87.82%和88.44%。细胞毒性实验结果显示,各siRNA转染未出现明显的细胞毒性(P>0.05),对细胞正常生理功能未产生影响,可用于后续实验。

|
| The main axis of the figure (left y axis) represents the relative expression of target genes detected by real-time fluorescent quantitative PCR (real-time PCR) (in RQ value). The secondary axis (right y axis) represents the cytoxicity of siRNAs (expressed as the cell survival rate normalized to the blank control group). CTRL: Caco-2 cells transfected without siRNA (blank control group). *P<0.05 (n=6). Error bar: SD. 图 1 siRNA的干扰效率与细胞毒性检测 Fig. 1 Interference efficiency and cytotoxicity of siRNAs |
将上述各目的基因siRNA转染Caco-2细胞,48 h后进行EV-A71感染实验。48 h后,采用免疫荧光法检测目的基因下调对EV-A71感染细胞的影响。结果显示,与对照组相比,ABCC3和SLC7A7基因下调明显抑制了EV-A71对Caco-2细胞的感染(见图 2A)。计算病毒量发现,ABCC3和SLC7A7基因下调对EV-A71感染细胞的抑制率分别达87.05%和81.66%,但其余目的基因下调没有明显抑制EV-A71对Caco-2细胞的感染(见图 2B)。
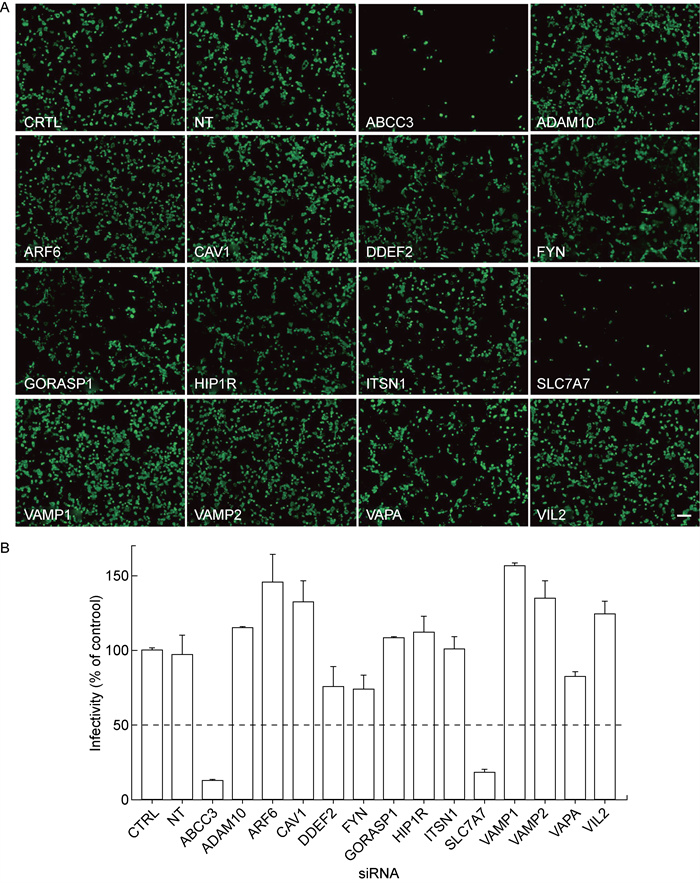
|
| A: The immunofluorescence results of EV-A71 in Caco-2 cells tansfected with siRNA of each target gene. B: The infectivity of EV-A71 in Caco-2 cells after down-regulation of each target gene. CTRL: Caco-2 cells transfected without siRNA (blank control group); NT: Caco-2 cells transfected with non-targeting siRNA (negative control group); Horizontal dotted line: the reference line for the viral infection rate of 50% relative to the blank control group. n=6. Error bar: SD. Scale bar=200 μm. 图 2 siRNA敲减目的基因对EV-A71感染细胞的影响 Fig. 2 Influence of siRNA interference on infection of EV-A71 in Caco-2 cells |
为进一步明确ABCC3和SLC7A7在EV-A71感染中的重要作用,将3条针对ABCC3和SLC7A7基因的siRNA分别转染Caco-2细胞,观察其对EV-A71感染性的影响。采用real-time PCR检测siRNA的干扰效率,real-time PCR和蛋白质印迹法分别检测EV-A71核酸与蛋白水平。结果显示,不同siRNA的干扰效率不同(见图 3A),其中ABCC3 siRNA(SEQ ID NO: 1)和SLC7A7 siRNA(SEQ ID NO: 29)的干扰效率分别高达87.82%和88.44%。3条ABCC3和3条SLC7A7基因的siRNA对EV-A71感染的抑制率均达50%以上,并且随着干扰效率增高,Caco-2细胞中EV-A71载量显著下降(P < 0.05)(见图 3B、3C)。
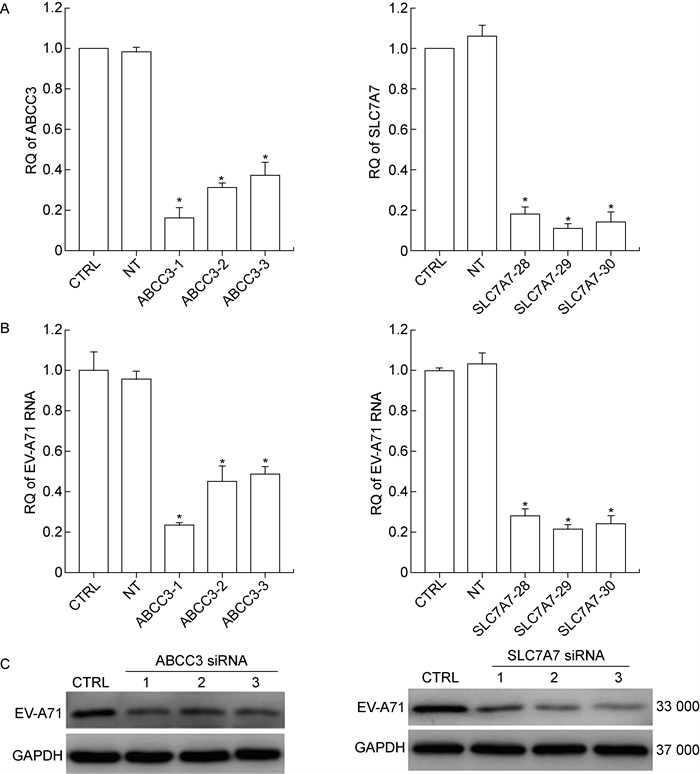
|
| A: mRNA level of target genes detected by real-time fluorescent quantitative PCR (real-time PCR) after transfection with siRNAs targeting ABCC3 and SLC7A7. B: mRNA level of EV-A71 detected by real-time PCR after transfection with siRNAs targeting ABCC3 and SLC7A7. C: EV-A71 VP1 protein expression detected by Western blotting after transfection with siRNAs targeting ABCC3 and SLC7A7. CTRL: Caco-2 cell transfected without siRNA (blank control group); NT: Caco-2 cell transfected with non-targeting siRNA (negative control group). *P<0.05 (n=6). Error bar: SD. 图 3 ABCC3和SLC7A7基因敲减对EV-A71复制的影响 Fig. 3 Influence of ABCC3 and SLC7A7 knockdown on replication of EV-A71 in Caco-2 cells |
EV-A71通过粪-口和(或)呼吸道飞沫传播,亦可经接触感染,其中以粪-口途径传播最广。在此传播途径中,肠道是人体抵御病原体的第1道屏障[24]。EV-A71经口腔接种小鼠后,最先在小肠组织中检测出病原体阳性。在EV-A71所致HFMD患者的粪便中可分离到病毒,且患者肠道功能受损[25-26]。随访调查发现,手足口病患儿中EV-A71感染越重其肠道排毒时间越长[27]。由此推断,EV-A71很可能首先感染肠道系统,在小肠细胞内大量快速复制,随后入血导致病毒血症并引发其他器官的感染。
由于Caco-2细胞具有与人小肠黏膜上皮细胞相似的形态学和生理学功能,本研究以Caco-2作为靶细胞,选择了一组宿主细胞转运相关膜蛋白进行筛选。通过检索NCBI GenBank数据库获得细胞转运相关膜蛋白ABCC3、ADAM10、ARF6、CAV1、DDEF2、FYN、GORASP1、HIP1R、ITSN1、SLC7A7、VAMP1、VAMP2、VAPA和VIL2的全序列和mRNA序列,利用网络资源及常用软件进行生物信息学分析,选择编码区作为靶序列设计siRNA,通过下调这些基因观察其对EV-A71感染的影响,进而找到可有效抑制EV-A71感染Caco-2细胞的宿主因子,即ABCC3和SLC7A7。
本研究结果表明,ABCC3和SLC7A7分子在EV-A71感染Caco-2细胞中发挥着重要作用,下调ABCC3和SLC7A7基因能显著抑制EV-A71感染。ABCC3属于ABC家族成员,在肝、肠道、胰腺、胆囊和肾中表达,可以转运葡糖苷酸、谷胱甘肽和硫酸盐等的结合物以及单阴离子胆汁酸。人类ABCC3通过转运依托泊苷、替尼泊苷和长春新碱等药物参与多种抗肿瘤药物的耐药过程,过表达ABCC3可以促进细胞增殖、耐药和有氧糖酵解,且与肿瘤进展和预后相关[28-30]。SLC7A7编码Y+L氨基酸转运蛋白1,这是一种不依赖钠的氨基酸转运蛋白,存在于上皮细胞膜,主要通过调节阳离子和大的中性氨基酸跨膜转运来调节多种生理功能。SLC7A7突变导致的阳离子转运蛋白功能障碍与多种临床症状相关,如高血氨、肾功能障碍、胃肠道症状、异常造血、生长迟缓和骨质疏松症[31]。SLC7A7的高表达与恶性胶质瘤和多发性骨髓瘤的预后、卵巢癌的耐药性以及肺癌的放射治疗敏感性高度相关[17, 32-33]。
本研究结果显示,ABCC3和SLC7A7基因下调不影响细胞正常生理功能,但明显抑制EV-A71对Caco-2细胞的感染。目前还未见任何关于ABCC3和SLC7A7在EV-A71感染宿主细胞中作用的报道。最新研究表明,ABCC3能够调控胆固醇的流动性,从而影响细胞膜上的囊泡运输[34]。我们前期研究结果显示,细胞膜胆固醇会参与流行性乙型脑炎病毒及EV-A71感染人神经细胞[35],本研究中ABCC3和SLC7A7通过何种机制参与EV-A71感染细胞、是否也通过影响胆固醇转运而参与EV-A71入侵正在进一步研究中。因此,对这两个分子进行深入研究不仅能够提升对EV-A71感染与致病机制的认识,还可以为预防与治疗EV-A71感染提供新的靶点与思路。
| [1] |
Solomon T, Lewthwaite P, Perera D, Cardosa MJ, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71[J]. Lancet Infect Dis, 2010, 10(11): 778-790.
[DOI]
|
| [2] |
孙建东, 陶臻. 肠道病毒71型抗病毒药物的最新研究进展[J]. 医学综述, 2020, 26(5): 980-985. [DOI]
|
| [3] |
王春荣. 肠道病毒71型感染手足口病发病机制的研究[J]. 世界华人消化杂志, 2019, 27(24): 1465-1472. [CNKI]
|
| [4] |
Chen YC, Yu CK, Wang YF, Liu CC, Su IJ, Lei HY. A murine oral enterovirus 71 infection model with central nervous system involvement[J]. J Gen Virol, 2004, 85(Pt 1): 69-77.
[URI]
|
| [5] |
Weldon RA Jr, Parker WB, Sakalian M, Hunter E. Type D retrovirus capsid assembly and release are active events requiring ATP[J]. J Virol, 1998, 72(4): 3098-3106.
[DOI]
|
| [6] |
Müller KH, Kainov DE, El Bakkouri K, Saelens X, De Brabander JK, Kittel C, Samm E, Muller CP. The proton translocation domain of cellular vacuolar ATPase provides a target for the treatment of influenza A virus infections[J]. Br J Pharmacol, 2011, 164(2): 344-357.
[DOI]
|
| [7] |
Carrasco-Torres G, Fattel-Fazenda S, López-Alvarez GS, García-Román R, Villa-Treviño S, Vásquez-Garzón VR. The transmembrane transporter ABCC3 participates in liver cancer progression and is a potential biomarker[J]. Tumour Biol, 2016, 37(2): 2007-2014.
[DOI]
|
| [8] |
Bleibaum F, Sommer A, Veit M, Rabe B, Andra J, Kunzelmann K, Nehls C, Correa W, Gutsmann T, Grotzinger J, Bhakdi S, Reiss1 K. ADAM10 sheddase activation is controlled by cell membrane asymmetry[J]. J Mol Cell Biol, 2019, 11(11): 979-993.
[DOI]
|
| [9] |
Duan X, Zhang HL, Pan MH, Zhang Y, Sun SC. Vesicular transport protein Arf6 modulates cytoskeleton dynamics for polar body extrusion in mouse oocyte meiosis[J]. Biochim Biophys Acta Mol Cell Res, 2018, 1865(2): 455-462.
[DOI]
|
| [10] |
Campos A, Salomon C, Bustos R, Díaz J, Martínez S, Silva V, Reyes C, Díaz-Valdivia N, Varas-Godoy M, Lobos-González L, Quest AF. Caveolin-1-containing extracellular vesicles transport adhesion proteins and promote malignancy in breast cancer cell lines[J]. Nanomedicine (Lond), 2018, 13(20): 2597-2609.
[DOI]
|
| [11] |
Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion[J]. Cancer Cell, 2003, 4(6): 499-515.
[DOI]
|
| [12] |
Kon S, Tanabe K, Watanabe T, Sabe H, Satake M. Clathrin dependent endocytosis of E-cadherin is regulated by the Arf6GAP isoform SMAP1[J]. Exp Cell Res, 2008, 314(7): 1415-1428.
[DOI]
|
| [13] |
Wang H, Yu LC, Li YC. Protein tyrosine kinase regulates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking induced by acute hypoxia in cultured brainstem neurons[J]. Genet Mol Res, 2016, 15(3).
[DOI]
|
| [14] |
Hu F, Shi X, Li B, Huang X, Morelli X, Shi N. Structural basis for the interaction between the Golgi reassembly-stacking protein GRASP65 and the Golgi matrix protein GM130[J]. J Biol Chem, 2015, 290(44): 26373-26382.
[DOI]
|
| [15] |
Jain RN, Al-Menhali AA, Keeley TM, Ren JH, El-Zaatari M, Chen XS, Merchant JL, Ross TS, Chew CS, Samuelson LC. Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice[J]. J Clin Invest, 2008, 118(7): 2459-2470.
[URI]
|
| [16] |
Pechstein A, Shupliakov O, Haucke V. Intersectin 1: a versatile actor in the synaptic vesicle cycle[J]. Biochem Soc Trans, 2010, 38(Pt 1): 181-186.
[URI]
|
| [17] |
Agnelli L, Forcato M, Ferrari F, Tuana G, Todoerti K, Walker BA, Morgan GJ, Lombardi L, Bicciato S, Neri A. The reconstruction of transcriptional networks reveals critical genes with implications for clinical outcome of multiple myeloma[J]. Clin Cancer Res, 2011, 17(23): 7402-7412.
[DOI]
|
| [18] |
IsenmannS, Khew-Goodall Y, Gamble J, Vadas M, Wattenberg BW. A splice-isoform of vesicle-associated membrane protein-1 (VAMP-1) contains a mitochondrial targeting signal[J]. Mol Biol Cell, 1998, 9(7): 1649-1660.
[DOI]
|
| [19] |
Hasan N, Hu C. Vesicle-associated membrane protein 2 mediates trafficking of alpha5beta1 integrin to the plasma membrane[J]. Exp Cell Res, 2010, 316(1): 12-23.
[DOI]
|
| [20] |
Wyles JP, McMaster CR, Ridgway ND. Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum[J]. J Biol Chem, 2002, 277(33): 29908-29918.
[DOI]
|
| [21] |
Jin YC, Han JA, Xu CX, Kang SK, Kim SH, Seo KS, Yoon DH, Choi YJ, Lee HG. Functional study of Villin 2 protein expressed in longissimus dorsi muscle of Korean native cattle in different growth stages[J]. BMB Rep, 2012, 45(2): 102-107.
[DOI]
|
| [22] |
Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation[J]. Am J Physiol Gastrointest Liver Physiol, 2004, 286(3): G367-G376.
[DOI]
|
| [23] |
王志勇, 吕延成. RNA干扰中小干扰RNA的设计原则[J]. 医学综述, 2010, 16(5): 675-677. [DOI]
|
| [24] |
陈生林, 朱勇喆, 戚中田. 肠道病毒71型复制周期研究进展[J]. 中国病毒病杂志, 2015, 5(2): 144-149. [CNKI]
|
| [25] |
Kahrs CR, Chuda K, Tapia G, Stene LC, Mårild K, Rasmussen T, Rønningen KS, Lundin KEA, Kramna L, Cinek O, Størdal K. Enterovirus as trigger of coeliac disease: nested case-control study within prospective birth cohort[J]. BMJ, 2019, 364: l231.
[URI]
|
| [26] |
王美芬, 陈韬, 顾涛, 罗云娇, 杜曾庆, 王明英. 不同肠道病毒感染手足口病患儿儿茶酚胺、S-100蛋白及D-乳酸水平变化及其临床意义[J]. 中国全科医学, 2017, 20(16): 1968-1972. [DOI]
|
| [27] |
Teng S, Wei Y, Zhao SY, Lin XY, Shao QM, Wang J. Intestinal detoxification time of hand-foot-and-mouth disease in children with EV71 infection and the related factors[J]. World J Pediatr, 2015, 11(4): 380-385.
[DOI]
|
| [28] |
Kobayashi K, Ito K, Takada T, Sugiyama Y, Suzuki H. Functional analysis of nonsynonymous single nucleotide polymorphism type ATP-binding cassette transmembrane transporter subfamily C member 3[J]. Pharmacogenet Genomics, 2008, 18(9): 823-833.
[DOI]
|
| [29] |
Liu X, Yao D, Liu C, Cao Y, Yang Q, Sun Z, Liu D. Overexpression of ABCC3 promotes cell proliferation, drug resistance, and aerobic glycolysis and is associated with poor prognosis in urinary bladder cancer patients[J]. Tumour Biol, 2016, 37(6): 8367-8374.
[DOI]
|
| [30] |
Fernández-Barrena MG, Monte MJ, Latasa MU, Uriarte I, Vicente E, Chang HC, Rodriguez-Ortigosa CM, Elferink RO, Berasain C, Marin JJ, Prieto J, Ávila MA. Lack of Abcc3 expression impairs bile-acid induced liver growth and delays hepatic regeneration after partial hepatectomy in mice[J]. J Hepatol, 2012, 56(2): 367-373.
[DOI]
|
| [31] |
Ji X, Yang X, Wang N, Kang M, Wang Y, Rong L, Fang Y, Xue Y. Function of SLC7A7 in T-Cell acute lymphoblastic leukemia[J]. Cell Physiol Biochem, 2018, 48(2): 731-740.
[DOI]
|
| [32] |
Fan S, Zhao Y, Li X, Du Y, Wang J, Song X, Zhou F, Chen H, Chen G, Zhao Y, Mao Y, Lan Q. Genetic variants in SLC7A7 are associated with risk of glioma in a Chinese population[J]. Exp Biol Med (Maywood), 2013, 238(9): 1075-1081.
[DOI]
|
| [33] |
Cheng L, Lu W, Kulkarni B, Pejovic T, Yan X, Chiang JH, Hood L, Odunsi K, Lin B. Analysis of chemotherapy response programs in ovarian cancers by the next-generation sequencing technologies[J]. Gynecol Oncol, 2010, 117(2): 159-169.
[DOI]
|
| [34] |
Järvinen E, Deng F, Kidron H, Finel M. Efflux transport of estrogen glucuronides by human MRP2, MRP3, MRP4 and BCRP[J]. J Steroid Biochem Mol Biol, 2018, 178: 99-107.
[DOI]
|
| [35] |
陈生林, 朱耐伟, 朱勇喆, 徐庆强, 彭浩然, 戚中田. 细胞膜胆固醇在流行性乙型脑炎病毒及肠道病毒71型感染人神经细胞中的作用[J]. 中国媒介生物学及控制杂志, 2016, 27(3): 253-256. [CNKI]
|
 2021, Vol. 16
2021, Vol. 16


