2. 内蒙古医科大学附属医院检验科,内蒙古 呼和浩特 010050;
3. 内蒙古自治区第四医院病理科,内蒙古 呼和浩特 010110;
4. 内蒙古医科大学附属医院儿科,内蒙古 呼和浩特 010050
2. Clinical Laboratory, Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010050, Inner Mongolia Autonomous Region, China;
3. Department of Pathology, The Fourth Hospital of Inner Mongolia, Hohhot 010110, Inner Mongolia Autonomous Region, China;
4. Department of Pediatrics, Affiliated Hospital of Inner Mongolia Medical University, Hohhot 010050, Inner Mongolia Autonomous Region, China
鲍曼不动杆菌(Acinetobacter baumannii,A. baumannii)作为机会性致病菌是战争伤的常见病原菌,易导致危重患者呼吸机相关性肺炎、脑膜炎和菌血症[1-2]。它是医院感染中多重耐药菌ESKAPE群的成员[3],尽管表现为低毒力[4],但对亚胺培南、美罗培南等抗生素明显耐药,致使感染者死亡率上升[5-7]。鲍曼不动杆菌通过形成生物膜、黏附和侵入细胞以及获取铁而致病,致病性依赖外膜蛋白等毒力因子,故认为其毒力和致病性随抗生素、致病因子和宿主免疫反应等多种条件而变化。本研究在评估携带不同毒力基因谱多重耐药鲍曼不动杆菌临床株的基础上,选用携带毒力基因多、致病力强的菌株构建免疫抑制小鼠肺炎模型,观察肺炎进程及炎症介质变化,旨在建立易操作、成本低、重复性好的小鼠肺炎模型,分析其感染或致病特征,为解释鲍曼不动杆菌肺炎的发病机制及致病原因奠定基础。
1 材料与方法 1.1 主要试剂与仪器主要试剂与仪器如下。DNA提取盒、引物、MasterMix、染料、DNA Marker购自天根生化科技(北京)有限公司;低熔点琼脂糖购自美国Genetech公司;TAE缓冲液购自Thermo公司;N2野生型秀丽隐杆线虫、尿嘧啶缺陷型大肠埃希菌OP50购自上海南方模式生物科技股份有限公司;线虫生长培养基(nematode growth medium,NGM)的配制参考相应文献[8]、线虫M9缓冲液、琼脂粉、蛋白胨购自Sino公司;环磷酰胺(cyclophosphamide,CTX)购自北京百灵威科技有限公司;舒泰购自北京宠必威生物科技有限公司;磷酸盐缓冲液(phosphate buffered saline,PBS)购自北京索莱宝科技有限公司;血平板购自天津市金章科技发展有限公司;细胞因子酶联免疫吸附试验(enzyme-linked immunosorbent assay,ELISA)试剂盒购自泉州市科诺迪生物科技有限公司;聚合酶链反应(polymerase chain reaction,PCR)仪购自北京领宇科技有限公司;电泳仪、Gel DocXR+凝胶成像分析系统购自北京六一生物科技有限公司;DENSIMAT麦氏比浊仪购自法国梅里埃公司;鱼跃超声雾化器420B购自江苏鱼跃医疗设备股份有限公司;迈瑞BC-5000全自动血细胞分析仪购自深圳迈瑞生物医疗电子股份有限公司;离心机、病理切片机HM325购自美国Thermo公司;Nexcope NE950正置荧光显微镜购自宁波永新光学股份有限公司;莱特体视显微镜LS745购自广州市莱特光电技术有限公司。
1.2 实验动物无特定病原体(specific pathogen free,SPF)级雄性BALB/c小鼠购自北京维通利华实验动物技术有限公司,6~8周龄,体重18~20 g,已通过动物福利伦理认证[许可证号:SCXK(京)2016-0006]。小鼠自由进食,饮无菌水,于温度18~25 ℃、湿度50%~70%、光照与黑暗交替12 h的环境中分笼饲养。饲养及实验于中国疾病预防控制中心实验动物中心完成。
1.3 实验菌株 1.3.1 菌株复核和耐药性检测鲍曼不动杆菌A4株分离自脑出血患儿脑脊液标本,A5株分离自病毒性脑炎患儿下呼吸道分泌物标本。采用micro-Typer MALDI-TOF质谱仪(江苏天瑞仪器股份有限公司)对A4株和A5株进行鉴定。依据CLSI M100-S28药敏指南,利用微量肉汤稀释法检测鲍曼不动杆菌对常用抗菌药物的敏感性。
1.3.2 毒力基因检测采用DNA试剂盒提取A4、A5株DNA。PCR扩增反应体系总体积为25 μL,包括MasterMix 12.5 μL、上游引物0.5 μL、下游引物0.5 μL、DNA模板1 μL、蒸馏水10.5 μL。引物序列、产物长度及反应条件[9]如表 1所示。将PCR产物进行1%琼脂糖凝胶电泳(180 V,20 min),用凝胶成像系统扫描分析,结果通过美国国家生物技术信息中心(National Center for Biotechnology Information,NCBI)网站Blast比对分析。
| Primer | Sequence (5′-3′) | Length (bp) | Reaction condition | |
| iutA | F | GGCTGGACATCATGGGAACTGG | 300 | Pre-denaturation at 95 ℃ for 4 min, denaturation at 95 ℃ for 50 s, annealing at 58 ℃ for 60 s, elongation at 72 ℃ for 45 s, 30 cycles, elongation at 72 ℃ for 8 min |
| R | CGTCGGGAACGGGTAGAATCG | |||
| FyuA | F | TGATTAACCCCGCGACGGGAA | 880 | |
| R | CGCAGTAGGCACGATGTTGTA | |||
| cvaC | F | CACACACAAACGGGAGCTGTT | 680 | |
| R | CTTCCCGCAGCATAGTTCCAT | |||
| draBC | F | GCTGGGCAGCAAACTGATAACTCTC | 750 | |
| R | CATCAAGCTGTTTGTTCGTCCGCCG | |||
| csfA | F | ACTCTGACTTGACTATTACC | 200 | |
| R | AGATGCAGTCTGGTCAAC | |||
| sfa/focDE | F | CTCCGGAGAACTGGGTGCATCTTAC | 410 | Pre-denaturation at 95 ℃ for 4 min, denaturation at 94 ℃ for 60 s, annealing at 56 ℃ for 45 s, elongation at 72 ℃ for 60 s, 34 cycles, elongation at 72 ℃ for 10 min |
| R | CGGAGGAGTAATTACAAACCTGGCA | |||
| ibeA | F | AGGCAGGTGTGCGCCGCGTAC | 170 | |
| R | TGGTGCTCCGGCAAACCATGC | |||
| papC | F | GACGGCTGTACTGCAGGGTGTGGCG | 328 | Pre-denaturation at 95 ℃ for 6 min, denaturation at 95 ℃ for 50 s, annealing at 58 ℃ for 70 s, elongation at 72 ℃ for 55 s, 34 cycles, elongation at 72 ℃ for 10 min |
| R | ATATCCTTTCTGCAGGGATGCAATA | |||
| PAI | F | GGACATCCTGTTACAGCGCGCA | 930 | |
| R | TCGCCACCAATCACAGCCGAAC |
将鲍曼不动杆菌接种于血平板,37 ℃培养18~24 h复苏,传代过夜。挑取血平板表面生长的菌落,充分混于50 mL体积分数为0.9% NaCl中,用比浊仪将菌液密度调至3 Mcfarland(109 CFU/mL),梯度稀释至1/10(108 CFU/mL)备用。
1.3.4 筛选建模菌株选取2株携带不同毒力基因的多重耐药鲍曼不动杆菌(A4和A5株)感染秀丽隐杆线虫,观察毒力基因差异是否影响菌株毒力,从而选取建模菌株,实验基本流程参考文献[28]。将20只小鼠随机分为2组,每组10只,所有小鼠连续2 d每日腹腔注射CTX 3 mg。3 d后,2组小鼠分别腹腔注射108 CFU/mL的A4、A5株菌液各0.5 mL,正常喂养7 d,选取致死剂量最大的菌株用于建模。
1.4 小鼠肺部感染模型制备将45只小鼠饲喂3 d适应环境后,连续2 d每只小鼠腹腔注射3 mg CTX,再饲喂3 d,建立免疫抑制,随机分为高密度(109 CFU/mL)组、低密度(108 CFU/mL)组与对照组,每组15只。小鼠腹腔注射舒泰1 mg/只麻醉后,放入30 cm×15 cm×20 cm的密闭塑料盒,垫高颈部,入口连接雾化管,出口连接活性炭收集残余气体。分别将高密度菌液、低密度菌液以及生理盐水(对照组)各50 mL置于雾化器中雾化30 min(小鼠平均呼吸频率120次/min,平均潮气量0.15 mL),速度2 mL/min,高、低密度组雾化吸入细菌接种量约分别为3×109 CFU/只、3×108 CFU/只,雾化后竖立小鼠。所有操作在生物安全柜中进行。
1.5 小鼠体重及状态监测每日正常饲喂小鼠并称重,间隔4 h观察小鼠精神、活动等情况,若死亡则记录并解剖。根据小鼠状态评分(0~-5分)。0分:正常,活动佳且健康;-1分:精神反应略差,被毛略粗糙;-2分:精神反应差,被毛粗糙,呼吸促,行动少;-3分:精神反应极差,嗜睡,被毛粗糙,行动很少,蜷缩,闭眼;-4分:濒死状态;-5分:死亡。
1.6 外周血常规指标及细胞因子检测于雾化后0、24、48、72和96 h,每组随机取3只小鼠行眼眶静脉丛采血2份,一份行全血细胞分析,另一份1 000 g离心20 min,取血清于-20 ℃冷藏,检测血清白细胞介素6(interleukin 6,IL-6)及肿瘤坏死因子α (tumor necrosis factor α,TNF-α)水平。
1.7 肺组织形态、细菌定量及肺组织苏木精-伊红(Hematoxylin-Eosin,HE)染色取血后将小鼠处死,无菌条件下取小鼠肺组织并观察,结扎右主支气管,将0.5 mL磷酸缓冲盐溶液(phosphate buffered saline, PBS)注入左肺缓慢冲洗3次,回收支气管肺泡灌洗液(bronchoalveolar lavage fluid,BALF)。将左肺剪碎,加2 mL PBS充分研磨,70 μm滤膜过滤,用无菌PBS进行10倍稀释。各取100 μL BALF及肺组织匀浆,均匀平涂于血平板,置37 ℃培养18 h,计菌落数。无菌操作条件下将小鼠右肺固定,石蜡包埋,连续5 μm切片,进行HE染色,镜检。
1.8 统计学方法使用SPSS 23.0进行统计学处理,实验结果以mean±SD表示,采用方差分析,P<0.05代表差异有统计学意义。
2 结果 2.1 A4株和A5株的耐药特点及毒力基因检测A4株和A5株对头孢曲松、头孢唑林、头孢吡肟、庆大霉素、妥布霉素、环丙沙星、阿米卡星、磺胺甲恶唑+甲氧苄啶(复方新诺明)、亚胺培南、美罗培南均耐药,均为多重耐药株;对替加环素均敏感。菌株毒力基因检测结果如表 2所示。
| Strain | Virulence gene | ||||||||
| iutA | FyuA | cvaC | draBC | csfA | sfa/focDE | ibeA | papC | PAI | |
| A4 | + | - | + | - | - | - | + | + | - |
| A5 | + | - | + | - | + | + | + | + | - |
用A4株和A5株感染秀丽隐杆线虫,结果显示,菌液密度为109 CFU/mL时,感染A5株的线虫于第5天出现死亡,感染A4株的线虫于第5天仅为僵直,于第9天出现死亡。菌液密度为108 CFU/mL时,感染A5株的线虫于第8天出现死亡,而感染A4株的线虫在观察期内无死亡。用相同菌液密度感染小鼠时,感染A4株的小鼠1 d后被毛粗糙,眼裂减小,刺激后活动差,7 d后死亡7只;而感染A5株的小鼠1 d后闭眼不动,呼吸快,嗜睡,7 d后均死亡(见表 3)。在同等菌量感染条件下,A5株致线虫死亡更早且小鼠死亡数最多,故选毒力更强的自下呼吸道分离的A5株作为建模菌株。
| Group | n | Total | Death rate (%) | ||||||
| 1 d | 2 d | 3 d | 4 d | 5 d | 6 d | 7 d | |||
| A4 | 3 | 2 | 2 | 0 | 0 | 0 | 0 | 7 | 70 |
| A5 | 6 | 3 | 1 | 0 | 0 | 0 | 0 | 10 | 100 |
感染A5株后小鼠的体重减轻。与对照组相比,从0 h起感染A5株的小鼠体重减轻,24 h达最低,之后回升,高密度组比低密度组体重减轻更为明显。感染A5株后小鼠状态变差:呼吸急促,被毛粗糙,反应迟钝,活动减少,24 h最差,之后好转;高密度组较低密度组状态更差,呈蜷缩,闭眼,濒死状态,并死亡1只(见图 1)。

|
| A: before immunization; B: 1 d after immunization; C: 2 d after immunization; D, E, F, G and H: 0, 24, 48, 72 and 96 h after infection. 图 1 A5株感染小鼠的体重及状态变化 Fig. 1 Changes in body weight and health state of mice infected with A5 strain |
健康小鼠肺组织光泽好,呈淡粉色(见图 2a)。免疫抑制后对照组小鼠肺组织略充血(见图 2A)。感染A5株后24 h,小鼠肺组织明显肿胀充血,颜色晦暗伴融合出血,高密度组肺边缘较低密度组肿胀严重,呈灰白色(见图 2B、2b)。随着感染进程延长,肺组织逐步好转,充血肿胀减轻,色泽恢逐渐复(见图 2c、2d、2e、2C、2D、2E)。
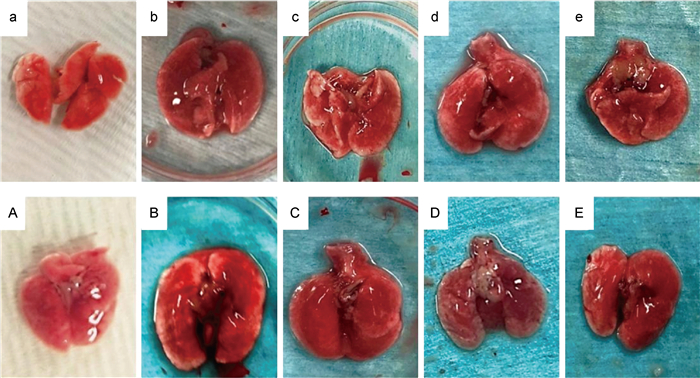
|
| a: healthy mice; A: control group; b, c, d and e: lung tissues of mice infected with A5 strain for 24, 48, 72 and 96 h in low density group; B, C, D, and E: lung tissues of mice infected with A5 strain for 24, 48, 72 and 96 h in high density group. 图 2 A5株感染小鼠的肺组织形态学特征 Fig. 2 Morphological characteristics of lung tissues in mice infected with A5 strain |
免疫抑制后小鼠肺支气管壁略厚(见图 3A)。感染后24、48 h肺支气管壁较对照组明显增厚,白细胞浸润,单核细胞渗出并间质充血水肿,高密度组小鼠肺组织炎性改变较低密度组更明显(见图 3b、3c、3B、3C);48 h后肺组织改变逐渐好转(见图 3d、3e、3D、3E)。

|
| a: healthy mice; A: control group; b, c, d and e: HE staining results of lung tissues of mice infected with A5 strain for 24, 48, 72 and 96 h in low density group; B, C, D, and E: HE staining results of lung tissues of mice infected with A5 strain for 24, 48, 72 and 96 h in high density group. 图 3 A5株感染小鼠的肺组织HE染色 Fig. 3 HE staining results of lung tissues in mice infected with A5 strain |
A5株感染小鼠后在BALF和肺组织内大量增殖,于0、24 h达高峰,均>5 logCFU/mL,显著高于对照组。感染早期,高密度组小鼠肺组织及BALF中CFU计数显著高于低密度组;随着时间推移,高、低密度组小鼠肺组织及BALF中CFU计数明显下降(见表 4)。
| Group | Intervention time | F | P value | |||||
| 0 h | 24 h | 48 h | 72 h | 96 h | ||||
| Control group | Lung tissue | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| BALF | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Low density group | Lung tissue | 5.23±0.09a | 5.35±0.03a | 5.13±0.04a | 0.00 | 0.00 | 1 231 082 | 0.00 |
| BALF | 5.56±0.05a | 5.12±0.12a | 4.82±0.19a | 0.00 | 0.00 | 16 078.407 | 0.00 | |
| High density group | Lung tissue | 5.34±0.02ab | 5.40±0.09ab | 4.52±0.07ab | 4.46±0.15ab | 0.00 | 473 626 | 0.00 |
| BALF | 5.65±0.06ab | 5.05±0.05ab | 4.73±0.05ab | 4.02±1.10ab | 0.00 | 15 050.919 | 0.00 | |
| aP<0.05 compared with the control group, bP<0.05 compared with low density group. | ||||||||
高、低密度组小鼠感染A5株后24 h外周血白细胞、中性粒细胞数开始增高,96 h达高峰,显著高于对照组;高密度组白细胞、中性粒细胞数显著高于低密度组(见表 5)。
| Group | Intervention time | F value | P value | |||||
| 0 h | 24 h | 48 h | 72 h | 96 h | ||||
| Healthy mice | Leukocyte | 4.87±1.91 | - | - | - | - | - | - |
| Neutrophil | 1.10±0.15 | - | - | - | - | - | - | |
| Control group | Leukocyte | 1.33±0.15 | 1.20±0.10 | 1.43±0.12 | 1.63±0.15 | 2.73±0.45 | 21.473 | 0.029 |
| Neutrophil | 0.50±0.10 | 0.47±0.06 | 0.50±0.10 | 0.80±0.10 | 1.20±0.36 | 0.966 | 0.049 | |
| Low density group | Leukocyte | 0.67±0.06a | 1.73±0.15a | 3.47±0.95 | 7.20±0.30a | 8.63±0.15a | 202.726 | 0.005 |
| Neutrophil | 0.27±0.06 | 0.43±0.06 | 0.60±0.20 | 1.20±0.10a | 1.43±0.12 | 81.893 | 0.006 | |
| High density group | Leukocyte | 0.80±0.10a | 1.73±0.15a | 3.27±1.55 | 8.10±1.00a | 12.73±0.55ab | 201.018 | 0.005 |
| Neutrophil | 0.47±0.15 | 0.53±0.06 | 0.50±0.20 | 1.67±0.06ab | 3.53±1.55ab | 9.563 | 0.090 | |
| F/P | Leukocyte | 30.545/0.001 | 15.059/0.005 | 3.405/0.103 | 99.183/0.000 | 429.113/0.000 | 78.630 | 0.000 |
| Neutrophil | 3.909/0.082 | 2.333/0.178 | 0.333/0.729 | 72.571/0.000 | 5.836/0.039 | 4.762 | 0.053 | |
| aP<0.05 compared with the control group, bP<0.05 compared with low density group. | ||||||||
与对照组相比,高、低密度组小鼠感染A5株后血清IL-6水平升高并趋向稳定;而TNF-α水平持续升高,低密度组在72 h达高峰,于96 h出现下降,高密度组在96 h达高峰,且低密度组TNF-α整体水平高于高密度组(见表 6)。
| Group | Intervention time | F value | P value | |||||
| 0 h | 24 h | 48 h | 72 h | 96 h | ||||
| Healthy mice | IL-6 | 3.35±0.02 | - | - | - | - | - | - |
| TNF-α | 13.80±2.80 | - | - | - | - | - | - | |
| Control group | IL-6 | 2.67±0.00 | 2.50±0.06 | 2.37±0.09 | 2.53±0.06 | 2.62±0.08 | 10.464 | 0.045 |
| TNF-α | 11.60±2.39 | 10.60±0.38 | 12.38±0.96 | 13.04±0.19 | 13.52±1.00 | 2.921 | 0.218 | |
| Low density group | IL-6 | 2.67±0.00 | 2.77±0.01a | 2.75±0.01a | 2.77±0.00a | 2.77±0.00a | 43.930 | 0.022 |
| TNF-α | 11.48±0.13 | 12.90±2.24 | 20.89±0.00a | 22.27±2.12a | 19.07±5.32 | 8.624 | 0.099 | |
| High density group | IL-6 | 2.68±0.00ab | 2.74±0.00a | 2.75±0.00a | 2.74±0.01a | 2.74±0.01a | 27.281 | 0.035 |
| TNF-α | 10.83±0.30 | 11.98±1.94 | 12.17±0.92b | 14.45±2.71b | 15.49±1.50 | 12.930 | 0.069 | |
| F/P | IL-6 | 39.803/0.000 | 47.379/0.000 | 49.889/0.000 | 36.915/0.000 | 8.507/0.018 | 14.382 | 0.001 |
| TNF-α | 0.270/0.772 | 1.356/0.327 | 124.829/0.000 | 18.738/0.003 | 2.260/0.186 | 4.558 | 0.032 | |
| aP<0.05 compared with the control group, bP<0.05 compared with low density group. | ||||||||
鲍曼不动杆菌在呼吸道等部位长期定植,常引起下呼吸道感染[10-11],其多重耐药特性极大增加了临床治疗的难度[12-13]。2021年CHINET监测网数据显示,鲍曼不动杆菌对亚胺培南和美罗培南的耐药率分别为71.5%和72.3%。本研究选取的鲍曼不动杆菌临床株对包括美罗培南、亚胺培南等在内的多种抗菌药物耐药。体外研究证实,鲍曼不动杆菌对呼吸道A549上皮细胞具有明显的毒力[14],还可刺激和活化巨噬细胞释放细胞因子[15-16]。因此,须建立一个动物模型探究鲍曼不动杆菌体内致病过程,以进一步阐明其感染机制。
鲍曼不动杆菌为条件性致病菌,常感染免疫力低下人群,免疫功能正常的动物不易感。因此,可通过注射CTX破坏细胞DNA、阻滞淋巴细胞增殖抑制小鼠免疫功能,从而增加建模成功率。本研究中,免疫抑制小鼠体重减低,外周血白细胞、中性粒细胞减少,但不影响其生存率,与Manepalli等[17]报道的一致。当然,免疫抑制会影响细菌对正常小鼠的毒力特征,因此本研究结果仅适用于免疫抑制的BALB/c小鼠。选择雾化吸入建立肺部感染模型出于如下考虑:经气道直接接种的方法可保证菌量相对精确[18],但创伤大,操作难;滴鼻法感染成功率高,但易造成小鼠误吸导致窒息[19]。本研究选用超声雾化,通过模拟机体吸入的过程来保证吸入肺部的菌量恒定。前期预实验选用不同密度的鲍曼不动杆菌感染小鼠,结果显示密度过高会导致小鼠过早死亡,达不到观察要求,而密度过低又很难使小鼠呈现感染状态。鉴于鲍曼不动杆菌为条件性致病菌,本研究选用108CFU/mL和109CFU/mL两个感染密度进行建模。
本研究在筛选鲍曼不动杆菌临床株时发现,分离自不同部位的菌株A4和A5均携带毒力基因cvaC、iutA、papC和ibeA,而csfA、sfa/focDE基因仅存在于A5株。研究显示,cvaC、papC和csfA与鲍曼不动杆菌菌毛形成相关,其中csfA是参与菌毛形成的主要亚基[20-22]。iutA通过铁载体需氧肌动蛋白参与鲍曼不动杆菌生物膜的合成[21],iutA及cvaC是鲍曼不动杆菌致病的主要非黏附性毒力基因[20-22]。sfa/focDE和ibeA则通过参与鲍曼不动杆菌定植及形成生物膜等过程增强其致病力[23]。本研究检测的9个毒力基因中,A5株毒力基因携带率高于A4株。将A4株和A5株感染线虫,发现高感染密度致线虫死亡早,密度相同时A5株致线虫死亡早,与小鼠致死性测定的结果一致。提示A5株毒力更强,故将A5株作为实验菌株。
本研究显示,感染A5株后小鼠体重较对照组明显减轻,精神状态变差,甚至死亡。小鼠肺组织于24 h呈明显充血水肿,且感染密度越高肺肿胀程度越重,与Yershov等[24]建立的肺部感染模型一致。小鼠肺组织及BALF中菌落计数均>1×105 CFU/mL,与既往研究相符[25]。小鼠的临床表现、病理结果及菌落计数结果充分表明,小鼠感染A5株可发生肺炎。但不足之处在于未进行小鼠外周血菌落计数,因而不能准确获得同时段外周血中A5株的分布特征。
同时,本研究利用外周血白细胞及中性粒细胞计数评价感染程度。感染A5株的小鼠白细胞及中性粒细胞计数在24 h较对照组升高,高密度组高于低密度组。结合小鼠肺及BALF中菌落计数发现,接种A5株后鲍曼不动杆菌显著增殖,小鼠肺内中性粒细胞聚集增多,释放大量的活性氧,造成广泛的组织损伤,与Lee等[26]的研究结果一致。在整个诱导免疫的过程中,小鼠感染A5株的密度越高,炎症程度越重,与菌株筛选时感染A5株的密度越高线虫死亡越早相一致。随着时间的延长,小鼠清除细菌的免疫应答能力逐步恢复,炎性细胞大量增加,肺及BALF中鲍曼不动杆菌被清除,小鼠肺部症状减轻,状态好转。
通过检测IL-6和TNF-α水平进一步评价小鼠感染A5株后的炎性反应。既往研究表明,衰老小鼠感染鲍曼不动杆菌野生株后72 h其IL-6水平降低[27],患者感染多重耐药鲍曼不动杆菌后IL-6水平更低[28]。TNF-α是机体受病原攻击后活化巨噬细胞分泌的可溶性细胞因子,通过各种途径间接刺激机体应答以增强机体对病原的反应能力。本研究基于免疫抑制,低密度组IL-6、TNF-α水平整体略高于高密度组,这可能是因为高密度组不仅激活肺吞噬细胞,还能调动血液免疫功能以清除细菌。同时,IL-6水平在略升高后趋于稳定,表明虽然感染可激发小鼠的免疫反应,但免疫抑制状态时白细胞的数量和活性低,感染后略有升高但不明显。但TNF-α水平有不同的变化趋势:低密度组小鼠外周血TNF-α水平明显升高,在感染后期出现下降,而高密度组则持续升高。高密度组在感染后期仍能检测到少量细菌存在,同时白细胞浸润现象更加明显。结合本研究中外周血白细胞数量、肺组织和BALF中菌量及肺部病理的特点发现,外周血TNF-α比IL-6能更好地动态评估鲍曼不动杆菌感染免疫抑制小鼠产生的免疫应答反应。
4 结语本研究选取来自下呼吸道标本的A5株,成功构建了免疫抑制小鼠肺部鲍曼不动杆菌感染模型,证明鲍曼不动杆菌可引起明显的肺部感染表现,提示来自下呼吸道标本的鲍曼不动杆菌临床株对免疫抑制小鼠具有较强的致病力,且感染程度与初始接种量有一定的相关性。因此,临床诊疗时,在免疫低下人群呼吸道标本中检出鲍曼不动杆菌时应给予更多的关注。
| [1] |
Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of acinetobacter infections: a century of challenges[J]. Clin Microbiol Rev, 2017, 30(1): 409-447.
[DOI]
|
| [2] |
Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities[J]. Clin Infect Dis, 2006, 42(5): 692-699.
[DOI]
|
| [3] |
Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen[J]. Clin Microbiol Rev, 2008, 21(3): 538-582.
[DOI]
|
| [4] |
Ushizawa H, Yahata Y, Endo T, Iwashima T, Misawa M, Sonobe M, Yamagishi T, Kamiya H, Nakashima K, Matsui T, Matsui M, Suzuki S, Shibayama K, Doi M, Irie F, Yamato S, Otomo Y, Oishi K. An epidemiological investigation of a nosocomial outbreak of multidrug-resistant Acinetobacter baumannii in a critical care center in Japan, 2011-2012[J]. Jpn J Infect Dis, 2016, 69(2): 143-148.
[DOI]
|
| [5] |
Sultan AM, Seliem WA. Identifying risk factors for healthcare-associated infections caused by carbapenem-resistant Acinetobacter baumannii in a neonatal intensive care unit[J]. Sultan Qaboos Univ Med J, 2018, 18(1): e75-e80.
[DOI]
|
| [6] |
Saipriya K, Swathi CH, Ratnakar KS, Sritharan V. Quorum-sensing system in Acinetobacter baumannii: a potential target for new drug development[J]. J Appl Microbiol, 2020, 128(1): 15-27.
[DOI]
|
| [7] |
Liu CP, Lu HP, Luor T. Clonal relationship and the association of the ST218 strain harboring blaOXA-72 gene to mortality in carbapenem-resistant Acinetobacter baumannii bacteremia[J]. J Microbiol Immunol Infect, 2019, 52(2): 297-303.
[DOI]
|
| [8] |
黄晓会, 徐阿晶, 俞静, 陈其琪, 费爱华, 卜书红. 耐药鲍曼不动杆菌感染秀丽隐杆线虫药物筛选模型的建立及应用[J]. 药学服务与研究, 2020, 20(02): 107-110. [DOI]
|
| [9] |
Askari N, Momtaz H, Tajbakhsh E. Acinetobacter baumannii in sheep, goat, and camel raw meat: virulence and antibiotic resistance pattern[J]. AIMS Microbiol, 2019, 5(3): 272-284.
[DOI]
|
| [10] |
Jain M, Sharma A, Sen MK, Rani V, Gaind R, Suri JC. Phenotypic and molecular characterization of Acinetobacter baumannii isolates causing lower respiratory infections among ICU patients[J]. Microb Pathog, 2019, 128: 75-81.
[DOI]
|
| [11] |
Vázquez-López R, Solano-Gálvez SG, Juárez Vignon-Whaley JJ, Abello Vaamonde JA, Padró Alonzo LA, Rivera Reséndiz A, Muleiro Álvarez M, Vega López EN, Franyuti-Kelly G, Álvarez-Hernández DA, Moncaleano Guzmán V, Juárez Bañuelos JE, Marcos Felix J, González Barrios JA, Barrientos Fortes T. Acinetobacter baumannii resistance: a real challenge for clinicians[J]. Antibiotics (Basel), 2020, 9(4): 205.
[DOI]
|
| [12] |
Chandenier J, Bernard S, Montharu J, Bailly E, Fetissof F, de Monte M, Desoubeaux G, Diot P, Richard-Lenoble D. The utility of a nebulised intra-tracheal rat model of invasive pulmonary aspergillosis[J]. Mycoses, 2009, 52(3): 239-245.
[DOI]
|
| [13] |
Pennington JE. Quantitative effects of immunosuppression on bronchoalveolar cells[J]. J Infect Dis, 1977, 136(1): 127-131.
[DOI]
|
| [14] |
马致洁, 于小红, 章从恩, 马晓晶, 王铁山, 李慧, 林龙飞, 赵奎君, 黄璐琦. 柴胡桂枝汤对泛耐药鲍曼不动杆菌侵袭的人肺癌A549细胞的保护作用[J]. 中国医院用药评价与分析, 2020, 20(11): 1281-1284. [DOI]
|
| [15] |
Sato Y, Unno Y, Miyazaki C, Ubagai T, Ono Y. Multidrug-resistant Acinetobacter baumannii resists reactive oxygen species and survives in macrophages[J]. Sci Rep, 2019, 9(1): 17462.
[DOI]
|
| [16] |
Sato Y, Unno Y, Ubagai T, Ono Y. Sub-minimum inhibitory concentrations of colistin and polymyxin B promote Acinetobacter baumannii biofilm formation[J]. PLoS One, 2018, 13(3): e0194556.
[DOI]
|
| [17] |
Manepalli S, Gandhi JA, Ekhar VV, Asplund MB, Coelho C, Martinez LR. Characterization of a cyclophosphamide-induced murine model of immunosuppression to study Acinetobacter baumannii pathogenesis[J]. J Med Microbiol, 2013, 62(Pt_11): 1747-1754.
[DOI]
|
| [18] |
张怡敏, 周雪宁, 张宏方, 环诚, 叶峥嵘. 小鼠鲍曼不动杆菌肺感染模型的制备[J]. 细胞与分子免疫学杂志, 2017, 33(10): 1392-1397. [DOI]
|
| [19] |
Knapp S, Wieland CW, Florquin S, Pantophlet R, Dijkshoorn L, Tshimbalanga N, Akira S, van der Poll T. Differential roles of CD14 and toll-like receptors 4 and 2 in murine Acinetobacter pneumonia[J]. Am J Respir Crit Care Med, 2006, 173(1): 122-129.
[DOI]
|
| [20] |
Mohajeri P, Sharbati S, Farahani A, Rezaei Z. Evaluate the frequency distribution of nonadhesive virulence factors in carbapenemase-producing Acinetobacter baumannii isolated from clinical samples in Kermanshah[J]. J Nat Sci Biol Med, 2016, 7(1): 58-61.
[DOI]
|
| [21] |
Fereshteh S, Abdoli S, Shahcheraghi F, Ajdary S, Nazari M, Badmasti F. New putative vaccine candidates against Acinetobacter baumannii using the reverse vaccinology method[J]. Microb Pathog, 2020, 143: 104114.
[DOI]
|
| [22] |
Duthy TG, Manning PA, Heuzenroeder MW. Identification and characterization of assembly proteins of CS5 pili from enterotoxigenic Escherichia coli[J]. J Bacteriol, 2002, 184(4): 1065-1077.
[DOI]
|
| [23] |
Wang S, Niu C, Shi Z, Xia Y, Yaqoob M, Dai J, Lu C. Effects of ibeA deletion on virulence and biofilm formation of avian pathogenic Escherichia coli[J]. Infect Immun, 2011, 79(1): 279-287.
[DOI]
|
| [24] |
Yershov AL, Jordan BS, Guymon CH, Dubick MA. Relationship between the inoculum dose of Streptococcus pneumoniae and pneumonia onset in a rabbit model[J]. Eur Respir J, 2005, 25(4): 693-700.
[DOI]
|
| [25] |
解岚岚, 王惠琴, 薛佩妮. 细菌性肺炎动物模型研究进展[J]. 徐州医科大学学报, 2017, 37(11): 769-772. [DOI]
|
| [26] |
Lee HH, Aslanyan L, Vidyasagar A, Brennan MB, Tauber MS, Carrillo-Sepulveda MA, Dores MR, Rigel NW, Martinez LR. Depletion of alveolar macrophages increases pulmonary neutrophil infiltration, tissue damage, and sepsis in a murine model of Acinetobacter baumannii pneumonia[J]. Infect Immun, 2020, 88(7): e00128-20.
[DOI]
|
| [27] |
Sato Y, Tansho-Nagakawa S, Ubagai T, Ono Y. Analysis of immune responses in Acinetobacter baumannii-infected klotho knockout mice: a mouse model of Acinetobacter baumannii infection in aged hosts[J]. Front Immunol, 2020, 11: 601614.
[DOI]
|
| [28] |
Chen J, Yang Y, Xiang K, Li D, Liu H. Combined rifampin and sulbactam therapy for multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia in pediatric patients[J]. J Anesth Perioper Med, 2018, 5(4): 176-185.
[DOI]
|
 2022, Vol. 17
2022, Vol. 17



