结核病是由结核分枝杆菌(Mycobacterium tuberculosis)感染引起的一种危害严重的传染病。卡介苗(Bacillus Calmette-Guérin,BCG)是将牛分枝杆菌毒株接种于人工培养基并连续传代而获得的减毒株,是目前唯一获准使用的预防结核病的疫苗。BCG接种不仅可预防儿童严重结核病,还可降低儿童非结核分枝杆菌感染引起的多种呼吸道疾病如肺炎、菌血症的发病率和死亡率。研究表明,BCG对其他细菌、病毒和寄生虫等非同源病原体感染也具有非特异性保护作用。此外,BCG作为非特异性免疫治疗剂,在临床上已用于一些肿瘤和自身免疫性疾病的治疗。BCG接种对多种病毒性感染具有非特异性保护作用[1]。流行病学研究表明,BCG疫苗接种与严重急性呼吸综合征冠状病毒2(severe acute respiratory syndrome coronavirus 2,SARS-CoV-2)感染引起的2019冠状病毒病(coronavirus disease 2019,COVID-19)的死亡率之间存在负相关[2-4]。其诱导的异源性保护被认为与一种天然免疫应答——训练免疫(trained immunity)有关。
1 训练免疫的定义训练免疫被定义为机体初次感染或疫苗接种后,天然免疫细胞发生表观遗传改变和代谢重编程,对同源或非同源再感染病原体产生更强的免疫应答[5-6]。训练免疫又称为天然免疫记忆(innate immunity memory)[7],其产生不依赖T/B细胞的适应性免疫[5],可同种异体转移[5, 8],也可遗传给下一代[9-10],并能更有效地激活适应性反应[11]。
训练免疫可被多种微生物因子诱导,包括细菌、病毒、真菌和寄生虫等微生物或病原体,如BCG、肺炎支原体、白假丝酵母、巨细胞病毒、黄热病毒、疟原虫,以及病原体组分如革兰阴性菌细胞壁成分脂多糖(lipopolysaccharide,LPS)、白假丝酵母的β -葡聚糖、分枝杆菌细胞壁成分胞壁酰二肽(muramyl dipeptide,MDP)和酿酒酵母的几丁质等[5, 7]。
2 BCG诱导的训练免疫机制训练免疫分子机制包括细胞代谢改变、表观遗传重编程以及对病原体再刺激产生的免疫应答,不同微生物因子诱导的反应有所不同[12]。BCG可通过激活天然免疫细胞表面模式识别受体(pattern recognition receptor,PRR)诱导细胞代谢改变,引起组蛋白表观遗传重编程。经BCG训练的免疫细胞PRR分子如Toll样受体4(Toll-like receptor 4,TLR4)、CD11b、CD14b、甘露糖受体等表达增加,再次感染时可迅速激活,诱导下游免疫应答[13-17]。BCG被吞噬和消化后释放MDP,MDP可与核苷酸结合寡聚化结构域2(nucleotide-binding oligomerization domain 2,NOD2)结合,改变组蛋白表观遗传修饰水平[15]。天然免疫细胞初次刺激后的表观遗传修饰变化使其保留“记忆”,从而对再感染迅速应答,并产生增强的免疫反应(见图 1)。
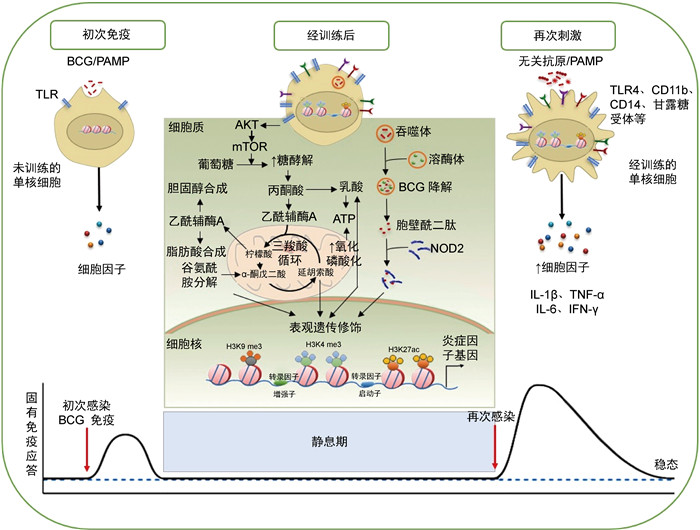
|
| 注:PAMP为病原相关分子模式(pathogen associated molecular pattern)。 图 1 BCG诱导的训练免疫机制图 Fig. 1 Schematic diagram depicting BCG-induced trained immunity |
BCG进入骨髓可“训练”造血干细胞(hematopoietic stem cell,HSC)[17]和多能祖细胞(multipotent progenitor,MPP)表观遗传重编程和染色质重组,产生表观遗传修饰的骨髓来源的巨噬细胞(bone marrow-derive macrophage,BMDM)[17]和单核/巨噬细胞[13],为再次感染提供保护力。BCG还可诱导自然杀伤细胞(natural killer,NK)[11, 18]、天然淋巴细胞(innate lymphoid cell,ILC)[19]、黏膜相关恒定T细胞(mucosa-associated invariant T cell,MAIT)[20]等的表观遗传和代谢重编程,对异源性刺激产生免疫应答。此外,BCG免疫可诱导中性粒细胞紧急增加,从而在新生儿败血症中表现出保护作用[21-22]。BCG接种健康人可诱导中性粒细胞中训练免疫激活标记增加,引起抗菌功能相关基因的表达增加[23]。此外,BCG接种也会影响辅助性T细胞Th1和Th17的免疫反应,表现为其对无关病原体产生的促炎细胞因子增加[11]。
2.2 BCG诱导的细胞代谢改变细胞代谢的变化是细胞表型和激活的重要驱动因素[24]。糖酵解增加是训练免疫靶细胞代谢改变的核心标志之一。BCG免疫诱导单核细胞中哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)基因的表达上调,该基因是低氧诱导因子1 α (hypoxia inducible factor-1 α,HIF-1 α)的靶标,可使细胞中的有氧糖酵解(Warburg效应)以mTOR/HIF-1 α依赖性方式长期增加[25]。BCG免疫还会增强单核细胞中三羧酸循环(tricarboxylic acid cycle,TCA;也称克雷布斯循环)的活性,导致代谢中间产物如柠檬酸、琥珀酸、α -酮戊二酸、延胡索酸等的积累。同时,BCG诱导的单核细胞耗氧率增加,提示氧化磷酸化增加[25]。经BCG训练的单核细胞中糖酵解和氧化磷酸化均增加,这体现了BCG诱导细胞代谢改变的独特模式[13]。
此外,13C-葡萄糖通量分析提示,BCG接种会诱导单核细胞中磷酸戊糖代谢增加[25]。BCG免疫激活TCA循环来促进乙酰辅酶A产生,引起甲羟戊酸累积,促进胆固醇合成[26]。甲羟戊酸可激活单核细胞的训练免疫,其抑制剂氟伐他汀可抑制BCG诱导的训练免疫[27]。谷氨酰胺酶抑制剂阻碍谷氨酰胺分解代谢,从而降低BCG诱导的训练免疫水平[28]。谷氨酰胺分解后为TCA循环提供了α -酮戊二酸,导致某些TCA循环代谢物如延胡索酸的积累,从而诱导训练免疫反应[25]。
BCG接种诱导天然免疫细胞代谢重排,产生的中间代谢物为表观遗传修饰酶提供了底物,或作为表观遗传“写入”或“擦除”共激活因子或共抑制因子,在训练免疫的发展和维持过程中起着至关重要的作用[29]。
2.3 BCG诱导的细胞表观遗传修饰变化BCG诱导的天然免疫细胞表观遗传修饰变化主要包括组蛋白修饰、非编码RNA和DNA甲基化水平变化,可调节相关基因表达的激活或抑制[1, 30]。
组蛋白修饰是训练免疫调节天然免疫细胞信号转导的重要分子机制[31]。BCG接种可诱导组蛋白乙酰化和甲基化修饰,通过调节免疫分子启动子和增强子区域的可及性,从而诱导或抑制细胞中相关基因的表达。BCG诱导的mTOR、糖酵解关键酶以及炎症因子启动子区H3K4 me3和H3K27ac表达显著增加,而H3K9 me3水平显著降低[25, 32-33]。BCG诱导组蛋白以NOD2依赖性方式上调H3K4 me3的修饰水平,诱导天然免疫细胞炎症因子和TLR4的表达,共同防御同源或异源病原微生物感染[13-17]。此外,炎症趋化因子簇上游主导lncRNA(upstream master lncRNA of the inflammatory chemokine locus,UMLILO)是一种新的超级增强子驻留lncRNA。H3K4 me3通过lncRNA定位于基因组中的特定启动子[31]。在BCG免疫的CD14+单核细胞中UMLILO的表达上调[34],提示lncRNA参与调控BCG诱导的训练免疫。
DNA甲基化水平可调节损伤、炎症反应和防御反应相关基因调控区染色质的可及性,从而调节基因的转录和表达。BCG接种对结核分枝杆菌再感染产生应答者的体内天然免疫细胞DNA甲基化具有明显的促进作用,增强了巨噬细胞杀灭结核分枝杆菌的能力,该过程与白细胞介素1 β (interleukin 1 β,IL-1 β)的产生有关[35]。有研究通过绘制BCG接种后不同时间外周血单核细胞甲基化差异基因图谱,鉴定出了43个甲基化差异基因,发现其与调节吞噬作用的肌动蛋白调节途径密切相关[35]。
2.4 BCG训练的天然免疫细胞对再感染的免疫应答在小鼠模型中,BCG接种诱导了单核/巨噬细胞的组蛋白表观遗传重编程,使细胞表面分子CD14、TLR4、CD11b、甘露糖受体等表达上调,再感染时可迅速活化下游信号通路,促进多种细胞因子释放,包括IL-1 β、IL-6、肿瘤坏死因子α (tumor necrosis factor α,TNF- α)、γ干扰素(interferon γ,IFN- γ)、IL-18等[14-17]。BCG免疫诱导的抗炎因子表达的表观遗传修饰水平依赖胞内NOD2通路激活[15]。采用BCG经呼吸道黏膜免疫恒河猴后,将支气管灌洗液中的细胞以结核分枝杆菌菌体成分刺激,可检测到IL-2和TNF- α分泌水平提高,而LPS再刺激后细胞因子分泌水平无显著变化[33]。用不同病原体裂解物刺激BCG接种婴儿的外周血单核细胞,发现11种细胞因子和趋化因子表达水平提高[36]。IL-1家族细胞因子和受体广泛感应和调节天然免疫应答,其中IL-1 β是训练免疫的特征性细胞因子[37]。成人接种BCG的相关研究表明,IL-1 β在BCG诱导的抵抗黄热病病毒减毒疫苗株感染中发挥了关键的作用[13]。此外,自噬抑制剂可阻断β -葡聚糖和BCG体外诱导训练免疫,表明自噬对BCG诱导的训练免疫具有关键作用[38-39]。
2.5 BCG诱导的训练免疫的持续时间BCG接种后诱导的表观遗传修饰分子标记随时间而逐渐衰减,但仍保持一定的水平。再刺激时,经过训练的天然免疫细胞可迅速活化,释放细胞因子,帮助机体抗感染[40]。但单核细胞等天然免疫细胞的寿命相对较短,并不能将它们的记忆特征传递下去。HSC是主要存在于骨髓中的长寿细胞。BCG可诱导HSC重新编程,并产生表观遗传修饰的巨噬细胞,从而提供持续的保护[17, 23]。动物实验表明,BCG皮下免疫小鼠可诱导NK细胞的训练免疫,且持续时间长达1年[41-42]。健康成人皮下接种BCG后,外周血单核细胞对结核分枝杆菌再感染的IFN- γ反应至少可持续3个月[17]。另据报道,BCG诱导的训练免疫在人体可至少持续1年[11]。BCG诱导的对结核分枝杆菌的保护作用可持续15~20年,此后逐渐减弱[43-44]。对欧洲BCG接种国家的COVID-19死亡人数进行分析,结果提示BCG接种诱导的训练免疫对异源性感染的保护作用可能持续很长时间(约20年)[45]。需要指出的是,BCG接种效果受多种因素的影响,如菌株[45]、接种途径[46-48]、个体昼夜节律[49]、性别[50]、接种年龄[36]、公共卫生政策[51]等。因此,对BCG诱导的训练免疫持续时间仍须进一步研究。
3 BCG对病毒感染的非特异性保护作用 3.1 BCG接种对各种病毒感染的非特异性作用多项临床研究表明,BCG接种对病毒感染具有非特异性保护作用[52]。一项临床试验对65岁以上老人出院时给予接种BCG,与安慰剂组相比,BCG接种组首次感染的时间推迟,且新感染发生率降低。BCG接种对呼吸道感染的影响最显著,尤其是病毒感染[53]。目前人体和动物实验均已证实,BCG诱导的训练免疫对人乳头瘤病毒、呼吸道合胞病毒、流感病毒、单纯疱疹病毒、肝炎病毒等感染均显示出保护作用。但BCG接种对这些病毒感染的反应机制有所不同,仍须进一步研究(详见表 1)[52]。
| 病毒 | 研究类型 | 效应 | |
| 人体研究 | 黄热病毒 | 随机对照试验 | 降低IL-1β产生相关的黄热病疫苗病毒滴度 |
| 人乳头瘤病毒 | 随机对照试验 | 提高病毒性疣的清除 | |
| 病例 | |||
| 呼吸道合胞病毒 | 病例-对照研究 | 与几内亚比绍儿童感染少量呼吸道合胞病毒没有关联 | |
| 甲型H1N1流感病毒 | 随机对照试验 | 增加抗体产生 | |
| 单纯疱疹病毒 | 病例 | 缩短临床单纯疱疹病毒感染发病期 | |
| 动物研究 | 单纯疱疹病毒1型 | CD-1小鼠 | 提高存活率 |
| 单纯疱疹病毒2型 | 未知 | 提高存活率和保护力 | |
| CD-1小鼠 | |||
| 甲型流感病毒 | 未知 | 降低甲型流感病毒滴度 | |
| C57BL/6小鼠 | 增加巨噬细胞的胞吞作用,减少炎症 | ||
| CD-1小鼠 | 提高存活率 | ||
| H7N9亚型禽流感病毒 | BALB/c小鼠 | 未增加保护力 | |
| 乙型肝炎病毒 | C57BL/6小鼠 | 增加抗体产生 | |
| 乙型脑炎病毒 | BALB/c小鼠 | 临床症状延迟,提高存活率 | |
| 脑心肌炎病毒 | C57BL/10小鼠 | 增加抵抗力(由非致病性结核分枝杆菌引发) | |
| 鼠痘病毒 | DDN小鼠 | 提高存活率,增加IFN-γ的产生 | |
| 牛痘病毒 | BALB/c小鼠 | 预防感染(由胞壁酰二肽引发) | |
| C57BL/6小鼠 | 预防感染,增加IFN-γ的产生 |
2020年,一项发表在medRxiv预印本平台的研究表明,长期、普遍接种BCG的国家人群中COVID-19死亡率比BCG未普遍接种的国家显著降低。流行病学研究表明,BCG接种与COVID-19的流行和死亡率之间存在负相关[2-4, 54]。线性混合模型显示,排除年龄、人均国内生产总值、人口密度、人口规模、净移民率和各种文化维度等因素的影响,BCG接种可诱导对COVID-19的保护力[45]。一项对加利福尼亚州洛杉矶6 679名医护人员的回顾性研究表明,BCG接种与COVID-19相关临床症状减少以及抗SARS-CoV血清阳性率降低有关[55]。但也有研究报道显示,在COVID-19大流行早期(2020年4月)BCG接种与COVID-19死亡率呈负相关,而2020年8月的数据分析发现两者之间的相关性消失[56]。以色列在20世纪80年代停止了BCG接种,比较BCG接种计划停止前后3年出生个体的COVID-19发病率,结果显示两者之间无显著差异[57]。截至目前,共有22项随机对照试验对BCG抗COVID-19的作用进行了研究,将为BCG是否能降低COVID-19的发病率和严重程度提供答案[56]。
4 结语近10年来,BCG诱导的非特异性保护力引起了研究者的关注,而BCG接种对SARS-CoV-2感染保护力的发现亦促进了训练免疫机制的研究[3, 55]。除BCG外,麻疹疫苗[58]和口服脊髓灰质炎疫苗[59-60]也被证明可防止异源性感染[61]。一种由多种灭活细菌组成的疫苗MV130经黏膜免疫可减少儿童哮喘[62]及成人病毒和(或)细菌感染发作的次数[63],并可提供抗SARS-CoV-2感染的保护力,提高COVID-19疫苗的效能[64],其机制也与训练免疫有关[63]。因此,对BCG诱导的训练免疫研究为开发其他感染性疾病疫苗的新用途提供了思路。例如:在未来新发病原体大流行而短期内无法获得特异性疫苗时,BCG等疫苗诱导的训练免疫可能提供一定的保护力,从而减轻新发病原体在人群中的流行[3, 55]。
此外,未来利用BCG诱导的训练免疫还应当考虑到,训练免疫可增强免疫应答,也可能加重已存在的炎症性疾病。如动脉粥样硬化中,低密度脂蛋白诱导单核/巨噬细胞代谢和表观遗传重编程,诱发了长期促炎表型,反而加速了疾病的进程[14]。因此,将BCG诱导的训练免疫用于疫苗研发,不仅要对现有免疫计划进行优化,还要考虑人群的免疫应答水平或基础疾病,通过良好的实验设计以避免可能带来的有害影响,并对总体效应进行缜密的评估分析,尽可能使BCG诱导的训练免疫发挥有益的效应。
| [1] |
Singh AK, Netea MG, Bishai WR. BCG turns 100: its nontraditional uses against viruses, cancer, and immunologic diseases[J]. J Clin Invest, 2021, 131(11): e148291.
[DOI]
|
| [2] |
Netea MG, Giamarellos-Bourboulis EJ, Domínguez-Andrés J, Curtis N, van Crevel R, van de Veerdonk FL, Bonten M. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 infection[J]. Cell, 2020, 181(5): 969-977.
[DOI]
|
| [3] |
Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG vaccination to reduce the impact of COVID-19[J]. Lancet, 2020, 395(10236): 1545-1546.
[DOI]
|
| [4] |
Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19)[J]. Proc Natl Acad Sci U S A, 2020, 117(30): 17720-17726.
[DOI]
|
| [5] |
Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense[J]. Cell Host Microbe, 2011, 9(5): 355-361.
[DOI]
|
| [6] |
Netea MG, Schlitzer A, Placek K, Joosten LAB, Schultze JL. Innate and adaptive immune memory: an evolutionary continuum in the host's response to pathogens[J]. Cell Host Microbe, 2019, 25(1): 13-26.
[DOI]
|
| [7] |
Rusek P, Wala M, Druszczyńska M, Fol M. Infectious agents as stimuli of trained innate immunity[J]. Int J Mol Sci, 2018, 19(2): 456.
[DOI]
|
| [8] |
Netea MG, van der Meer JW. Trained immunity: an ancient way of remembering[J]. Cell Host Microbe, 2017, 21(3): 297-300.
[DOI]
|
| [9] |
Boutros M, Agaisse H, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila[J]. Dev Cell, 2002, 3(5): 711-722.
[DOI]
|
| [10] |
Kurtz J. Memory in the innate and adaptive immune systems[J]. Microbes Infect, 2004, 6(15): 1410-1417.
[DOI]
|
| [11] |
Kleinnijenhuis J, Quintin J, Preijers F, Benn CS, Joosten LA, Jacobs C, van Loenhout J, Xavier RJ, Aaby P, van der Meer JW, van Crevel R, Netea MG. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity[J]. J Innate Immun, 2014, 6(2): 152-158.
[DOI]
|
| [12] |
Tercan H, Riksen NP, Joosten LAB, Netea MG, Bekkering S. Trained immunity: long-term adaptation in innate immune responses[J]. Arterioscler Thromb Vasc Biol, 2021, 41(1): 55-61.
[PubMed]
|
| [13] |
Arts RJW, Moorlag SJCFM, Novakovic B, Li Y, Wang SY, Oosting M, Kumar V, Xavier RJ, Wijmenga C, Joosten LAB, Reusken CBEM, Benn CS, Aaby P, Koopmans MP, Stunnenberg HG, van Crevel R, Netea MG. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity[J]. Cell Host Microbe, 2018, 23(1): 89-100.
[DOI]
|
| [14] |
Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Baßler K, Klee K, Schulte-Schrepping J, Ulas T, Moorlag SJCFM, Kumar V, Park MH, Joosten LAB, Groh LA, Riksen NP, Espevik T, Schlitzer A, Li Y, Fitzgerald ML, Netea MG, Schultze JL, Latz E. Western diet triggers NLRP3-dependent innate immune reprogramming[J]. Cell, 2018, 172(1/2): 162-175.
|
| [15] |
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes[J]. Proc Natl Acad Sci U S A, 2012, 109(43): 17537-17542.
[DOI]
|
| [16] |
Walachowski S, Tabouret G, Fabre M, Foucras G. Molecular analysis of a short-term model of beta-glucans-trained immunity highlights the accessory contribution of GM-CSF in priming mouse macrophages response[J]. Front Immunol, 2017, 8: 1089.
[DOI]
|
| [17] |
Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier JC, Mailhot-Léonard F, Ahmed E, Belle J, Besla R, Mazer B, King IL, Nijnik A, Robbins CS, Barreiro LB, Divangahi M. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis[J]. Cell, 2018, 172(1/2): 176-190.
|
| [18] |
Pyzik M, Gendron-Pontbriand EM, Vidal SM. The impact of Ly49-NK cell-dependent recognition of MCMV infection on innate and adaptive immune responses[J]. J Biomed Biotechnol, 2011, 2011: 641702.
[DOI]
|
| [19] |
Steigler P, Daniels NJ, McCulloch TR, Ryder BM, Sandford SK, Kirman JR. BCG vaccination drives accumulation and effector function of innate lymphoid cells in murine lungs[J]. Immunol Cell Biol, 2018, 96(4): 379-389.
[DOI]
|
| [20] |
Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection[J]. Infect Immun, 2012, 80(9): 3256-3267.
[DOI]
|
| [21] |
Brook B, Harbeson DJ, Shannon CP, Cai B, He D, Ben-Othman R, Francis F, Huang J, Varankovich N, Liu A, Bao W, Bjerregaard-Andersen M, Schaltz-Buchholzer F, Sanca L, Golding CN, Larsen KL, Levy O, Kampmann B, Consortium E, Tan R, Charles A, Wynn JL, Shann F, Aaby P, Benn CS, Tebbutt SJ, Kollmann TR, Amenyogbe N. BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis[J]. Sci Transl Med, 2020, 12(542): eaax4517.
[DOI]
|
| [22] |
Brook B, Schaltz-Buchholzer F, Ben-Othman R, Kollmann T, Amenyogbe N. A place for neutrophils in the beneficial pathogen-agnostic effects of the BCG vaccine[J]. Vaccine, 2022, 40(11): 1534-1539.
[DOI]
|
| [23] |
Moorlag SJCFM, Rodriguez-Rosales YA, Gillard J, Fanucchi S, Theunissen K, Novakovic B, de Bont CM, Negishi Y, Fok ET, Kalafati L, Verginis P, Mourits VP, Koeken VACM, de Bree LCJ, Pruijn GJM, Fenwick C, van Crevel R, Joosten LAB, Joosten I, Koenen H, Mhlanga MM, Diavatopoulos DA, Chavakis T, Netea MG. BCG vaccination induces long-term functional reprogramming of human neutrophils[J]. Cell Rep, 2020, 33(7): 108387.
[DOI]
|
| [24] |
Murray PJ, Rathmell J, Pearce E. SnapShot: Immunometabolism[J]. Cell Metab, 2015, 22(1): 190-190.el.
[DOI]
|
| [25] |
Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, Kleinnijenhuis J, Lachmandas E, Goncalves LG, Belinha A, Cunha C, Oosting M, Joosten LAB, Matarese G, van Crevel R, Netea MG. Immunometabolic pathways in BCG-induced trained immunity[J]. Cell Rep, 2016, 17(10): 2562-2571.
[DOI]
|
| [26] |
Arts RJ, Joosten LA, Netea MG. Immunometabolic circuits in trained immunity[J]. Semin Immunol, 2016, 28(5): 425-430.
[DOI]
|
| [27] |
Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden CDCC, Li Y, Popa CD, Ter Horst R, van Tuijl J, Netea-Maier RT, van de Veerdonk FL, Chavakis T, Joosten LAB, van der Meer JWM, Stunnenberg H, Riksen NP, Netea MG. Metabolic induction of trained immunity through the mevalonate pathway[J]. Cell, 2018, 172(1/2): 135-146.
|
| [28] |
Arts RJ, Novakovic B, Ter Horst R, Carvalho A, Bekkering S, Lachmandas E, Rodrigues F, Silvestre R, Cheng SC, Wang SY, Habibi E, Goncalves LG, Mesquita I, Cunha C, van Laarhoven A, van de Veerdonk FL, Williams DL, van der Meer JW, Logie C, O'Neill LA, Dinarello CA, Riksen NP, van Crevel R, Clish C, Notebaart RA, Joosten LA, Stunnenberg HG, Xavier RJ, Netea MG. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity[J]. Cell Metab, 2016, 24(6): 807-819.
[DOI]
|
| [29] |
Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention[J]. Oncogene, 2007, 26(37): 5310-5318.
[DOI]
|
| [30] |
Verma D, Parasa VR, Raffetseder J, Martis M, Mehta RB, Netea M, Lerm M. Anti-mycobacterial activity correlates with altered DNA methylation pattern in immune cells from BCG-vaccinated subjects[J]. Sci Rep, 2017, 7(1): 12305.
[DOI]
|
| [31] |
Khader SA, Divangahi M, Hanekom W, Hill PC, Maeurer M, Makar KW, Mayer-Barber KD, Mhlanga MM, Nemes E, Schlesinger LS, van Crevel R, Vankayalapati R, Xavier RJ, Netea MG; Bill and Melinda Gates Foundation Collaboration for TB Vaccine Discovery Innate Immunity Working Group18. Targeting innate immunity for tuberculosis vaccination[J]. J Clin Invest, 2019, 129(9): 3482-3491.
[DOI]
|
| [32] |
Arts RJ, Blok BA, Aaby P, Joosten LA, de Jong D, van der Meer JW, Benn CS, van Crevel R, Netea MG. Long-term in vitro and in vivo effects of gamma-irradiated BCG on innate and adaptive immunity[J]. J Leukoc Biol, 2015, 98(6): 995-1001.
[DOI]
|
| [33] |
Vierboom MPM, Dijkman K, Sombroek CC, Hofman SO, Boot C, Vervenne RAW, Haanstra KG, van der Sande M, van Emst L, Domínguez-Andrés J, Moorlag SJCFM, Kocken CHM, Thole J, Rodríguez E, Puentes E, Martens JHA, van Crevel R, Netea MG, Aguilo N, Martin C, Verreck FAW. Stronger induction of trained immunity by mucosal BCG or MTBVAC vaccination compared to standard intradermal vaccination[J]. Cell Rep Med, 2021, 2(1): 100185.
[DOI]
|
| [34] |
Fanucchi S, Fok ET, Dalla E, Shibayama Y, Börner K, Chang EY, Stoychev S, Imakaev M, Grimm D, Wang KC, Li G, Sung WK, Mhlanga MM. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments[J]. Nat Genet, 2019, 51(1): 138-150.
[DOI]
|
| [35] |
Das J, Verma D, Gustafsson M, Lerm M. Identification of DNA methylation patterns predisposing for an efficient response to BCG vaccination in healthy BCG-naive subjects[J]. Epigenetics, 2019, 14(6): 589-601.
[DOI]
|
| [36] |
Smith SG, Kleinnijenhuis J, Netea MG, Dockrell HM. Whole blood profiling of bacillus Calmette-Guérin-induced trained innate immunity in infants identifies epidermal growth factor, IL-6, platelet-derived growth factor-AB/BB, and natural killer cell activation[J]. Front Immunol, 2017, 8: 644.
[DOI]
|
| [37] |
Moorlag SJCFM, Röring RJ, Joosten LAB, Netea MG. The role of the interleukin-1 family in trained immunity[J]. Immunol Rev, 2018, 281(1): 28-39.
[DOI]
|
| [38] |
Joosten LA, Netea MG, Dinarello CA. Interleukin-1beta in innate inflammation, autophagy and immunity[J]. Semin Immunol, 2013, 25(6): 416-424.
[DOI]
|
| [39] |
Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages[J]. Cell, 2004, 119(6): 753-766.
[DOI]
|
| [40] |
Gupta PK. New disease old vaccine: is recombinant BCG vaccine an answer for COVID-19?[J]. Cell Immunol, 2020, 356: 104187.
[DOI]
|
| [41] |
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Jacobs C, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. BCG-induced trained immunity in NK cells: role for non-specific protection to infection[J]. Clin Immunol, 2014, 155(2): 213-219.
[DOI]
|
| [42] |
Kleinnijenhuis J, van Crevel R, Netea MG. Trained immunity: consequences for the heterologous effects of BCG vaccination[J]. Trans R Soc Trop Med Hyg, 2015, 109(1): 29-35.
[DOI]
|
| [43] |
Nguipdop-Djomo P, Heldal E, Rodrigues LC, Abubakar I, Mangtani P. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: a retrospective population-based cohort study[J]. Lancet Infect Dis, 2016, 16(2): 219-226.
[DOI]
|
| [44] |
Abubakar I, Pimpin L, Ariti C, Beynon R, Mangtani P, Sterne JA, Fine PE, Smith PG, Lipman M, Elliman D, Watson JM, Drumright LN, Whiting PF, Vynnycky E, Rodrigues LC. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis[J]. Health Technol Assess, 2013, 17(37): 1-372.
[PubMed]
|
| [45] |
Gursel M, Gursel I. Is global BCG vaccination-induced trained immunity relevant to the progression of SARS-CoV-2 pandemic?[J]. Allergy, 2020, 75(7): 1815-1819.
[DOI]
|
| [46] |
Spencer JC, Ganguly R, Waldman RH. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with bacille Calmette-Guérin[J]. J Infect Dis, 1977, 136(2): 171-175.
[DOI]
|
| [47] |
Dijkman K, Sombroek CC, Vervenne RAW, Hofman SO, Boot C, Remarque EJ, Kocken CHM, Ottenhoff THM, Kondova I, Khayum MA, Haanstra KG, Vierboom MPM, Verreck FAW. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques[J]. Nat Med, 2019, 25(2): 255-262.
[DOI]
|
| [48] |
Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH 2nd, Hughes TK, Pokkali S, Swanson PA 2nd, Grant NL, Rodgers MA, Kamath M, Causgrove CM, Laddy DJ, Bonavia A, Casimiro D, Lin PL, Klein E, White AG, Scanga CA, Shalek AK, Roederer M, Flynn JL, Seder RA. Prevention of tuberculosis in macaques after intravenous BCG immunization[J]. Nature, 2020, 577(7788): 95-102.
[DOI]
|
| [49] |
de Bree LCJ, Mourits VP, Koeken VA, Moorlag SJ, Janssen R, Folkman L, Barreca D, Krausgruber T, Fife-Gernedl V, Novakovic B, Arts RJ, Dijkstra H, Lemmers H, Bock C, Joosten LA, van Crevel R, Benn CS, Netea MG. Circadian rhythm influences induction of trained immunity by BCG vaccination[J]. J Clin Invest, 2020, 130(10): 5603-5617.
[DOI]
|
| [50] |
Koeken VA, de Bree LCJ, Mourits VP, Moorlag SJ, Walk J, Cirovic B, Arts RJ, Jaeger M, Dijkstra H, Lemmers H, Joosten LA, Benn CS, van Crevel R, Netea MG. BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner[J]. J Clin Invest, 2020, 130(10): 5591-5602.
[DOI]
|
| [51] |
Fu W, Ho PC, Liu CL, Tzeng KT, Nayeem N, Moore JS, Wang LS, Chou SY. Reconcile the debate over protective effects of BCG vaccine against COVID-19[J]. Sci Rep, 2021, 11(1): 8356.
[DOI]
|
| [52] |
Moorlag SJCFM, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections[J]. Clin Microbiol Infect, 2019, 25(12): 1473-1478.
[DOI]
|
| [53] |
Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Dominguez-Andres J, Kyriazopoulou E, Gkavogianni T, Adami ME, Damoraki G, Koufargyris P, Karageorgos A, Bolanou A, Koenen H, van Crevel R, Droggiti DI, Renieris G, Papadopoulos A, Netea MG. Activate: randomized clinical trial of BCG vaccination against infection in the elderly[J]. Cell, 2020, 183(2): 315-323.
[DOI]
|
| [54] |
Covián C, Retamal-Díaz A, Bueno SM, Kalergis AM. Could BCG vaccination induce protective trained immunity for SARS-CoV-2?[J]. Front Immunol, 2020, 11: 970.
[DOI]
|
| [55] |
Rivas MN, Ebinger JE, Wu M, Sun N, Braun J, Sobhani K, Van Eyk JE, Cheng S, Arditi M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers[J]. J Clin Invest, 2021, 131(2): e145157.
[DOI]
|
| [56] |
Lindestam Arlehamn CS, Sette A, Peters B. Lack of evidence for BCG vaccine protection from severe COVID-19[J]. Proc Natl Acad Sci U S A, 2020, 117(41): 25203-25204.
[DOI]
|
| [57] |
Hamiel U, Kozer E, Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults[J]. JAMA, 2020, 323(22): 2340-2341.
[DOI]
|
| [58] |
Aaby P, Martins CL, Garly ML, Balé C, Andersen A, Rodrigues A, Ravn H, Lisse IM, Benn CS, Whittle HC. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial[J]. BMJ, 2010, 341: c6495.
[DOI]
|
| [59] |
Faridi MM, Srivastava S. Effect of simultaneous administration of oral polio vaccine on local reaction of BCG vaccine in term infants[J]. Indian Pediatr, 2015, 52(2): 115-118.
[DOI]
|
| [60] |
Lund N, Andersen A, Hansen AS, Jepsen FS, Barbosa A, Biering-Sørensen S, Rodrigues A, Ravn H, Aaby P, Benn CS. The effect of oral polio vaccine at birth on infant mortality: a randomized trial[J]. Clin Infect Dis, 2015, 61(10): 1504-1511.
[DOI]
|
| [61] |
Goodridge HS, Ahmed SS, Curtis N, Kollmann TR, Levy O, Netea MG, Pollard AJ, van Crevel R, Wilson CB. Harnessing the beneficial heterologous effects of vaccination[J]. Nat Rev Immunol, 2016, 16(6): 392-400.
[DOI]
|
| [62] |
Nieto A, Mazón A, Nieto M, Calderón R, Calaforra S, Selva B, Uixera S, Palao MJ, Brandi P, Conejero L, Saz-Leal P, Fernández-Pérez C, Sancho D, Subiza JL, Casanovas M. Bacterial mucosal immunotherapy with MV130 Prevents recurrent wheezing in children: a randomized, double-blind, placebo-controlled clinical trial[J]. Am J Respir Crit Care Med, 2021, 204(4): 462-472.
[DOI]
|
| [63] |
Brandi P, Conejero L, Cueto FJ, Martínez-Cano S, Dunphy G, Gómez MJ, Relaño C, Saz-Leal P, Enamorado M, Quintas A, Dopazo A, Amores-Iniesta J, Del Fresno C, Nistal-Villán E, Ardavín C, Nieto A, Casanovas M, Subiza JL, Sancho D. Trained immunity induction by the inactivated mucosal vaccine MV130 protects against experimental viral respiratory infections[J]. Cell Rep, 2022, 38(1): 110184.
[DOI]
|
| [64] |
Del Fresno C, García-Arriaza J, Martínez-Cano S, Heras-Murillo I, Jarit-Cabanillas A, Amores-Iniesta J, Brandi P, Dunphy G, Suay-Corredera C, Pricolo MR, Vicente N, López-Perrote A, Cabezudo S, González-Corpas A, Llorca O, Alegre-Cebollada J, Garaigorta U, Gastaminza P, Esteban M, Sancho D. The bacterial mucosal immunotherapy MV130 protects against SARS-CoV-2 infection and improves COVID-19 vaccines immunogenicity[J]. Front Immunol, 2021, 12: 748103.
[DOI]
|
 2022, Vol. 17
2022, Vol. 17


