2. 皖南医学院第一附属医院感染科, 芜湖 241000;
3. 复旦大学上海医学院医学分子病毒学重点实验室, 上海 200032;
4. 复旦大学附属静安区中心医院感染科, 上海 200040
2. Department of Infectious Diseases, the First Affiliated Hospital of Wannan Medical College, Wuhu 241000;
3. Key Laboratory of Medical Molecular Virology, Shanghai Medical College, Fudan University, Shanghai 200032, China;
4. Department of Infectious Diseases, Jing'an Branch of Huashan Hospital, Fudan University, Shanghai 200040, China
髓源性抑制细胞(myeloid-derived suppressor cells,MDSCs)是一群病理激活的、表型异常并具有免疫抑制功能的髓系来源细胞[1]。MDSCs在健康人体内含量有限,但在进展期肿瘤、败血症、慢性感染等病理环境下大量扩增,并通过多种途径抑制CD4+T细胞、CD8+T细胞、自然杀伤细胞(natural killer cell,NKC)等多种免疫细胞的增殖、活化和迁移,MDSCs的数量与多种疾病的进程和患者转归密切相关[1-3]。
近15年来,国内外MDSCs相关研究报道超过5 000篇,涉及领域广泛。新近研究表明:MDSCs和正常髓系细胞[包括中性粒细胞(neutrophils,NEU)等多核髓系细胞以及单核细胞(monocytes,MNC)等单核髓系细胞]在表型标记分子、基因表达、能量代谢以及调控通路上存在明显差异[4-6]。基于此,学界对MDSCs的定义和表型标记策略进行了相应的更新[7]。此外,在多种疾病特别是肿瘤领域,MDSCs靶向治疗研究也取得了一定的成果。鉴于此,笔者以MDSCs研究里程碑为起点,重点介绍MDSCs在表型标记分子、能量代谢、调控通路以及靶向治疗研究中的新近成果,并深入讨论急、慢性感染性疾病中MDSCs的功能亚群及相关调控通路。
1 MDSCs研究的里程碑1978年,Bennett等[8]发现,卡介苗注射可诱导小鼠骨髓中产生一群具有T细胞抑制作用的细胞,并将该群细胞命名为“自然抑制细胞(natural suppressor cells, NSCs)”。2000年始,Almand等[9]发现,终末期肿瘤患者体内富集一群表型异常的“未成熟髓系细胞”(immature myeloid cells,IMCs),该群细胞数量与患者免疫抑制程度呈正相关。随后,该群“未成熟髓系细胞”[或称为髓系抑制细胞(myeloid suppressor cells,MSCs)]的免疫抑制功能在肿瘤等多个领域被广泛证实。2007年,Gabrilovich等[10]建议将该群细胞以“MDSCs”统一命名,以强调其“髓系来源”和“免疫抑制”两大基本特征,这一建议得到了学界的广泛认同。2016年始,Condamine等[4]和Veglia等[5]运用单细胞测序、质谱流式等新技术,发现多核型MDSCs(polymorphonuclear MDSCs,PMN-MDSCs)与传统NEU在表型分子、基因表达和代谢特征上存在明显差异,从而拉开了MDSCs研究的新篇章,研究里程碑如图 1所示。
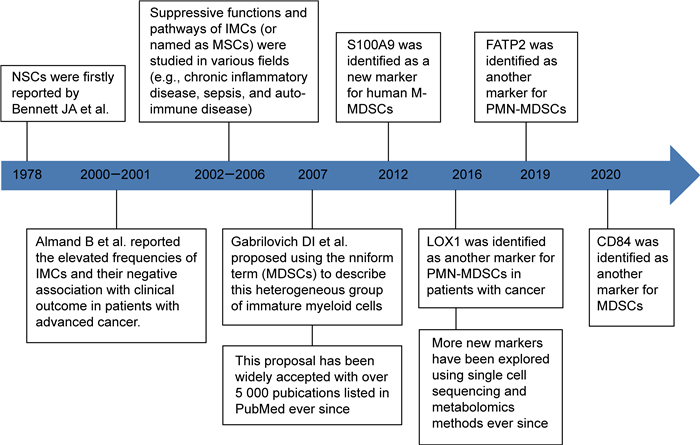
|
| Note: S100A9 (Ca-binding protein S100A9), LOX1 (lectin-type oxidized LDL receptor 1), FATP2 (fatty acid transporter protein 2), NSCs (natural suppressor cells), IMCS(immature myeloid cells), MSCs(myeloid suppressor cells), MDSCs(myeloid-derived suppressor cells). 图 1 MDSCs研究里程碑 Fig. 1 Milestones in MDSCs research |
当前MDSCs主要包括两大亚群——PMN-MDSCs和单核型MDSCs(monocytic MDSCs,M-MDSCs)。传统的MDSCs表型标记分子包括:①HLA-DR、CD11b或CD33、CD14和CD15(人);②Gr1、Ly6G和Ly6C(小鼠),如表 1所示。亦有研究表明:人体内存在部分成熟度更低的髓系细胞(Lin-HLA-DR-CD33+),该群细胞同样具有免疫抑制功能,从而被命名为早期型MDSCs(early MDSCs,E-MDSCs)[11]。但人体内E-MDSCs数量极少,而小鼠体内亦无对应的细胞亚群。近年来,随着单细胞测序和质谱流式等新技术的推广,一些新的MDSCs靶标分子被陆续发现,主要包括:S100A9[12]、凝集素型氧化低密度脂蛋白受体1(lectin-type oxidized LDL receptor 1,LOX1)[4]、脂肪酸转运蛋白2(fatty acid transport protein 2,FATP2)[5]和CD84[13]。基于此,学界对于MDSCs的表型标记策略进行了相应的更新(见表 1)。
| PMN-MDSCs | M-MDSCs | |
| 来源 | 普通髓系细胞;粒细胞前体细胞 | 普通髓系细胞;单核细胞前体细胞 |
| 经典表型标记策略 | CD11b+ CD14- CD15+ /CD66b+(人) Gr1+ Ly6G+ Ly6Clow/neg(小鼠) |
CD14+ CD15- HLA-DRlow/neg(人) Gr1+ Ly6G-Ly6Chigh(小鼠) |
| 新型表型标记策略(人) | CD15+/CD66b+ CD14- LOX1+ CD15+ /CD66b+ CD14- CD84+ |
CD14+ /CD66b- CXCR1+ CD14+ /CD66b- CD84+ CD14+S100A9high |
| 诱导因素 | 高浓度粒细胞-巨噬细胞集落刺激因子;血管内皮生长因子;白介素6;白介素1β;内质网应激 | 高浓度巨噬细胞集落刺激因子;血管内皮生长因子;白介素6;低氧诱导因子1α |
| 调控通路 | 信号转导和转录激活因子1/3/6;核转录因子κB;内质网应激;丝裂原激活的蛋白激酶;环氧合酶2;前列腺素E合酶 | 信号转导和转录激活因子3;钙结合蛋白S100A8/9;血管内皮生长因子 |
| 免疫抑制介质 | 活性氧自由基;精氨酸酶1;前列腺素E2 | 一氧化氮;转化生长因子1β;白介素1β;白介素10;程序性细胞死亡配体1 |
MDSCs可通过多种途径发挥免疫抑制作用,主要包括:①代谢剥夺,即通过分泌过量精氨酸酶-1(arginase-1,Arg-1)等代谢酶降解T细胞代谢所需的氨基酸;②氧化机制,即通过分泌过量活性氧自由基(reactive oxygen species,ROS)或一氧化氮(nitric oxide,NO)导致T细胞受体亚硝酸化;③细胞禁锢,即通过上调T细胞表面的抑制性分子表达(如L-选择素),或通过细胞间接触降低NKC上受体分子的表达(如NKp30受体),阻碍免疫细胞的迁移和发育;④负向诱导,即促进调节性T细胞活化[1, 7]。在肿瘤、慢性感染性疾病等持续性病理环境中,高浓度细胞生长因子、炎性细胞因子和内质网应激是MDSCs的主要诱导因素[7]。尽管PMN-MDSCs和M-MDSCs都具有免疫抑制功能,但两者在表型标记分子、诱导因素及调控通路、免疫抑制介质上存在明显差异(见表 1)。
3 MDSCs的基因特征已有研究表明,PMN-MDSCs与正常NEU比较,表达明显上调的基因涉及的信号通路包括:细胞周期、自噬、G蛋白信号、核转录因子蛋白κB(nuclear factor kappa-B,NF-κB)下游通路,以及白介素(interleukin,IL)-1β)、肿瘤坏死因子(tumour necrosis factor,TNF)等炎性细胞因子通路[14-15]。Condamine和Gabrilovich[16]发现:与健康人体内NEU相比,肿瘤患者体内PMN-MDSCs中的NF-κB、内质网应激和丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)等信号通路相关基因的表达明显上调。该团队的后续研究进一步显示:肿瘤患者体内PMN-MDSCs中脂质代谢相关基因(如LOX1和FATP2)的表达水平明显高于正常NEU[4-5]。Sasidharan等[17]发现:结肠癌患者体内PMN-MDSCs多种基因表达水平明显高于单核类抗原递呈细胞,涉及的通路包括:DNA损伤应答、趋化、凋亡、MAPK以及信号转导和转录激活因子(signal transducer and activator of transcription,STAT)。
已有研究表明:肿瘤患者体内M-MDSCs和MNC的基因表达呈现明显差异,主要表现为CXC型趋化因子受体(CXC chemokine receptor,CXCR)-1和STAT3的表达水平上调[16, 18]。Alshetaiwi等[13]发现:①乳腺癌患者体内MDSCs的基因表达明显区别于正常的NEU和MNC;②PMN-MDSCs和M-MDSCs比较,两者表达上调的基因存在一定重叠(包括IL-1β、Arg-2和CD84);③CD84是肿瘤患者体内MDSCs的新型标记分子,CD84highMDSCs能分泌更多ROS,而PMN-MDSCs同时呈现CD84和LOX1的高表达。
4 MDSCs的代谢特征对免疫细胞代谢所需氨基酸(如精氨酸、色氨酸和半胱氨酸)的“代谢剥夺”,是MDSCs重要的免疫抑制途径。2004年,Rodriguez[19]等首次报道了:肿瘤微环境中的PMN-MDSCs能够通过分泌Arg-1分解精氨酸,导致T细胞功能障碍。这已被多项相关研究所证实。亦有研究表明:M-MDSCs能够通过分泌诱导型一氧化氮合酶2(inducible nitric oxide synthase,INOS2)实现“精氨酸剥夺”[20-21],以及通过分泌吲哚胺2, 3-双加氧酶(indoleamine2, 3-dioxygenase,IDO)实现“色氨酸剥夺”[22-23]。
脂质代谢异常是MDSCs诱导分化过程的重要特征之一。已有研究表明:与正常的食饲小鼠相比,高脂食饲小鼠体内髓系前体细胞向MDSCs分化的程度、MDSCs的免疫抑制功能均增强;荷瘤小鼠体内MDSCs可通过上调清道夫受体CD36以摄取多不饱和脂肪酸等脂质,使其能量代谢方式由糖酵解向脂肪酸氧化转变[24-25]。新近研究表明:①PMN-MDSCs上FATP2的表达水平明显高于正常NEU;②PMN-MDSCs可通过上调FATP2的表达水平以摄取花生四烯酸并分泌前列腺素E2,从而抑制T细胞等免疫细胞的增殖与活化;③选择性FATP2抑制剂能够有效阻断PMN-MDSCs的免疫效应、抑制肿瘤进展,其联合检查点抑制剂可取得更好的治疗效果[5]。
此外,与正常的NEU或MNC相比,MDSCs糖代谢方式也存在差异。已有研究表明:在分化和迁移的过程中,MDSCs对葡萄糖和谷氨酰胺的摄取增多,耗氧量下降,糖酵解成为MDSCs主要的糖代谢方式[26];尤其在组织缺氧的环境中,MDSCs糖代谢由氧化磷酸化向糖酵解转换,而缺氧诱导因子1α表达水平上调是MDSCs糖代谢方式转变的重要因素[27]。但Baumann等[28]研究表明:肝细胞癌(hepatocellular carcinoma,HCC)组织中M-MDSCs的糖代谢和线粒体呼吸水平呈下降表现。
5 MDSCs的靶向治疗机制MDSCs特别是PMN-MDSCs在肿瘤细胞转移前微环境的形成,以及循环肿瘤细胞(circulating tumour cells,CTCs)在逃逸免疫杀伤和转移的过程中,发挥了重要的作用,其作用主要包括:①微环境改造;②免疫抑制;③网捕;④分泌ROS;⑤造成组织细胞炎症损伤[7]。CTCs自转移前微环境释放入循环系统再至靶器官病灶的过程中,MDSCs亦起到了重要作用,主要包括:①包裹在CTCs周围形成稳定的细胞簇,以逃逸NKC、CD8+T细胞等免疫杀伤细胞的攻击;②过量分泌ROS和Arg-1,以抑制免疫杀伤细胞的增殖与活化;③高表达基质金属蛋白酶和网捕CTCs,以利于CTCs转移至靶器官病灶[7]。
鉴于以上分析,MDSCs靶向治疗机制主要分为以下3个层面:①通过拮抗CD33等MDSCs特征性标记分子以消耗MDSCs[29-30],或通过阻断MDSCs诱导分化因素(如内质网应激)以抑制MDSCs的生成[31-32],或通过使用肝X受体激动剂以促进MDSCs的凋亡[33];②通过选择性抑制剂或基因工程下调MDSCs上CXCR-2、CXCR-4等趋化因子的表达水平,以抑制MDSCs的迁移[34-35];③使用FATP2抑制剂和环氧化酶2(cyclooxygenase 2,COX2)抑制剂等重塑MDSCs的能量代谢方式,以削弱其免疫抑制功能[5, 36]。
6 MDSCs与感染性疾病MDSCs与多种感染性疾病的疾病进程及患者转归存在密切关联。在急、慢性感染性疾病中,MDSCs的数量和优势亚群取决于病原种类、疾病阶段及机体免疫应答特征等多种因素。
6.1 MDSCs与急性感染性疾病MDSCs的数量与败血症的进展存在密切关联。Darcy等[37]研究表明:循环血PMN-MDSCs数量与败血症病情程度及患者体内的IL-6浓度呈正相关,PMN-MDSCs通过“精氨酸剥夺”抑制T细胞增殖。Janols等[38]通过比较不同病原败血症患者体内的MDSCs水平和亚群构成,发现革兰氏阳性菌败血症患者循环血中PMN-MDSCs扩增最为明显。Uhel等[39]和Ruan等[40]研究表明:与M-MDSC相比,循环血PMN-MDSCs数量与败血症患者免疫耐受状态的相关性更高,PMN-MDSCs持续富集更有助于预测患者发生二次感染的风险。Makarenkova等[41]研究表明:在盲肠结扎穿刺后12~24 h,小鼠入脾血流中PMN-MDSCs数量即显著升高,循环血中PMN-MDSCs数量与小鼠存活的时间呈负相关。但亦有研究表明:一定水平的PMN-MDSCs能够降低败血症模型小鼠体内的系统性炎症水平,从而延长小鼠的生存时间[42]。这可能与败血症的造模方式(盲肠结扎长度不易准确控制)导致的疾病严重程度不同有关。
MDSCs能够抑制宿主体内过强的细胞免疫应答,从而导致细菌或寄生虫感染的持续或慢性化。Poe等[43]通过研究克雷伯杆菌肺炎模型小鼠发现:循环血中MDSCs大量扩增并富集于模型小鼠肺脏,MDSCs能够通过分泌IL-10来抑制病灶中效应T细胞的活化,从而减少组织损伤。Arocena等[44]通过研究锥虫病模型小鼠发现:IL-6敲除或氟尿嘧啶处理的模型小鼠体内炎症水平和组织损伤明显高于对照组小鼠。Sanmarco等[45]研究表明:锥虫病模型小鼠体内MDSCs能够通过分泌NO促使T细胞受体亚硝酸化,抑制效应CD8+T细胞的免疫功能,从而降低小鼠体内的炎症水平和组织损伤。
6.2 MDSCs与慢性感染性疾病MDSCs介导的病原特异性T细胞活化障碍,是慢性感染性疾病发生与维持的重要原因。但在不同类型的慢性感染以及同一疾病的不同阶段,MDSCs的亚群构成和具体的免疫抑制途径存在一定的差异。
已有研究表明:活动性结核患者体内富集MDSCs,外周血或浆膜腔积液中MDSCs的数量与结核菌的数量呈正相关,MDSCs可通过分泌Arg-1、INOS2和IL-10抑制T细胞的增殖、活化,以促进结核菌在体内播散;PMN-MDSCs的数量可用于评估结核病的活动性和严重程度[46-47]。新近研究表明:使用白喉毒素重组蛋白[48]、COX2抑制剂[49]、全反式视黄酸[50]以及小分子抑制剂[51],能够有效降低结核感染模型小鼠体内的MDSCs水平,从而控制结核病的进展。
多项研究表明:HIV-1感染者体内PMN-MDSCs的水平与疾病进展和抗逆转录病毒药物疗效密切相关;PMN-MDSCs能够通过上调PD-L1和IL-4受体α的表达水平,或通过细胞接触下调T细胞表面CD3ζ的表达水平,以维持HIV-1感染和削弱抗病毒疗效[52-53]。亦有研究表明:HIV-1感染者体内存在M-MDSCs富集,但M-MDSCs数量与疾病进展的相关性尚不明确[54]。在其他类型的慢性病毒感染环境中,MDSCs的优势亚群通常是M-MDSCs。已有研究表明:M-MDSCs富集与EB病毒感染的慢性化和相关恶性肿瘤的发生密切相关[55-56];淋巴细胞性脉络丛脑膜炎病毒模型小鼠体内富集的M-MDSCs,能够有效抑制病原特异性T细胞的增殖和干扰素的分泌[57-58];慢性丙型肝炎病毒(hepatitis C virus,HCV)感染者体内富集M-MDSCs,其数量与HCV RNA水平呈正相关[59-60]。
在慢性乙型肝炎病毒(hepatitis B virus,HBV)感染者体内,M-MDSCs亦是MDSCs的主要功能亚群,其可通过上调PD-L1的表达水平、激活STAT3信号通路等途径,抑制感染者体内T细胞的增殖以及IFN-γ等功能性细胞因子的分泌[61-63]。Fang等[62]研究表明:慢性HBV感染者体内富集M-MDSCs,循环血中M-MDSCs的水平与乙肝表面抗原(hepatitis B surface antigen,HBsAg)水平呈正相关,且HBsAg可通过ERK/IL-6/STAT3轴诱导M-MDSCs生成。Yang等[63]研究表明:慢性HBV感染者循环血中M-MDSCs的水平与乙肝e抗原(hepatitis B envelope antigen,HBeAg)水平亦呈正相关,HBeAg可通过IL-6+IL-1β/IDO轴诱导M-MDSCs生成。但Pallett等[64]研究表明:免疫耐受期的慢性HBV感染者体内PMN-MDSCs的水平明显高于其他3个自然史时期(免疫清除期、免疫控制期、再活动期)的慢性HBV感染者;PMN-MDSCs可通过精氨酸代谢剥夺抑制T细胞活化及其对肝细胞造成的免疫损伤。
当慢性HBV感染进展到HBV相关HCC(HBV-related HCC, HBV-HCC)或HBV相关慢加急性肝衰竭(HBV-related acute-on-chronic liver failure,HBV-ACLF)阶段,患者体内MDSCs数量增加,同时MDSCs亚群构成亦可能发生变化。与慢性HBV感染者和健康对照相比,HBV-HCC患者体内PMN-MDSCs水平明显升高[65-66]。Nan等[65]发现:在HBV-HCC患者体内富集LOX1+NEU,该群细胞具有与PMN-MDSCs相似的免疫抑制功能和抑制介质;内质网应激是LOX1+NEU诱导分化的重要因素。Zeng等[67]研究表明:与慢性HBV感染者和健康对照相比,HBV-ACLF患者循环血中M-MDSCs的水平升高;且循环血M-MDSCs水平与HBV-ACLF进展以及患者短期死亡风险呈正相关。本课题组成员的一项研究(待发表)表明:HBV-ACLF患者体内M-MDSCs和PMN-MDSCs的水平均高于慢性乙肝患者和健康对照,但循环血PMN-MDSCs水平与继发细菌感染以及患者转归的相关性更为明显。新近研究表明:重构的曲美他嗪(Livantra)能够通过调节肝细胞能量代谢的方式(脂肪酸氧化—糖酵解),提高肝细胞内的ATP水平,减轻肝组织损伤和白细胞浸润,从而降低HBV-ACLF患者的短期死亡率[68]。
7 结语MDSCs和正常髓系细胞在表型标记、基因调控和能量代谢上存在显著差异,其中能量代谢方式转变在MDSCs分化、迁移和免疫抑制的过程中发挥了重要作用。单细胞测序、质谱流式等新技术的推广,为深入研究MDSCs的相关特征和靶向治疗提供了有利条件。但新近MDSCs研究成果多与肿瘤相关,部分研究结果也存在一定的差异。因此,有必要在慢性感染性疾病等非肿瘤领域中深入研究MDSCs的关键特征,从而为优化疾病诊疗措施和研发相关药物提供理论依据。
| [1] |
Hegde S, Leader A, Merad M. MDSC: Markers, development, states, and unaddressed complexity[J]. Immunity, 2021, 54(5): 875-884.
[DOI]
|
| [2] |
Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression[J]. Nat Rev Nephrol, 2018, 14(2): 121-137.
[DOI]
|
| [3] |
Lian M, Selmi C, Gershwin M, Ma X. Myeloid cells and chronic liver disease: a comprehensive review[J]. Clin Rev Allergy Immunol, 2018, 54(2): 307-317.
[DOI]
|
| [4] |
Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, Partlova S, Garfall A, Vogl DT, Xu X, Knight SC, Malietzis G, Lee GH, Eruslanov E, Albelda SM, Wang X, Mehta JL, Bewtra M, Rustgi A, Hockstein N, Witt R, Masters G, Nam B, Smirnov D, Sepulveda MA, Gabrilovich DI. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients[J]. Sci Immunol, 2016, 1(2): aaf8943.
[DOI]
|
| [5] |
Veglia F, Tyurin V, Blasi M, De Leo A, Kossenkov A, Donthireddy L, To T, Schug Z, Basu S, Wang F, Ricciotti E, DiRusso C, Murphy M, Vonderheide R, Lieberman P, Mulligan C, Nam B, Hockstein N, Masters G, Guarino M, Lin C, Nefedova Y, Black P, Kagan V, Gabrilovich D. Fatty acid transport protein 2 reprograms neutrophils in cancer[J]. Nature, 2019, 569(7754): 73-78.
[DOI]
|
| [6] |
Ostrand-Rosenberg S, Beury DW, Parker KH, Horn LA. Survival of the fittest: how myeloid-derived suppressor cells survive in the inhospitable tumor microenvironment[J]. Cancer Immunol Immunother, 2020, 69(2): 215-221.
[DOI]
|
| [7] |
Veglia F, Sanseviero E, Gabrilovich D. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity[J]. Nat Rev Immunol, 2021, 21(8): 485-498.
[DOI]
|
| [8] |
Bennett JA, Rao VS, Mitchell MS. Systemic bacillus Calmette-Guérin (BCG) activates natural suppressor cells[J]. Proc Natl Acad Sci, 1978, 75(10): 5142-5144.
[DOI]
|
| [9] |
Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer[J]. Clin Cancer Res, 2000, 6(5): 1755-1766.
[PubMed]
|
| [10] |
Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells[J]. Cancer Res, 2007, 67(1): 425.
[DOI]
|
| [11] |
Bronte V, Brandau S, Chen S, Colombo M, Frey A, Greten T, Mandruzzato S, Murray P, Ochoa A, Ostrand-Rosenberg S, Rodriguez P, Sica A, Umansky V, Vonderheide R, Gabrilovich D. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards[J]. Nat Commun, 2016(7): 12150.
[DOI]
|
| [12] |
Zhao F, Hoechst B, Duffy A, Gamrekelashvili J, Fioravanti S, Manns MP, Greten TF, Korangy F. S100A9 a new marker for monocytic human myeloid-derived suppressor cells[J]. Immunology, 2012, 136(2): 176-183.
[DOI]
|
| [13] |
Alshetaiwi H, Pervolarakis N, McIntyre LL, Ma D, Nguyen Q, Rath JA, Nee K, Hernandez G, Evans K, Torosian L, Silva A, Walsh C, Kessenbrock K. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics[J]. Sci Immunol, 2020, 5(44): eaay6017.
[DOI]
|
| [14] |
Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice[J]. J Leukoc Biol, 2012, 91(1): 167-181.
[DOI]
|
| [15] |
Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, Horng W, Fridlender G, Bayuh R, Worthen GS, Albelda SM. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils[J]. PLoS One, 2012, 7(2): e31524.
[DOI]
|
| [16] |
Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function[J]. Trends Immunol, 2011, 32(1): 19-25.
[DOI]
|
| [17] |
Sasidharan Nair V, Saleh R, Toor SM, Alajez NM, Elkord E. Transcriptomic analyses of myeloid-derived suppressor cell subsets in the circulation of colorectal cancer patients[J]. Front Oncol, 2020, 10: 1530.
[DOI]
|
| [18] |
Mastio J, Condamine T, Dominguez G, Kossenkov AV, Donthireddy L, Veglia F, Lin C, Wang F, Fu S, Zhou J, Viatour P, Lavilla-Alonso S, Polo AT, Tcyganov EN, Mulligan C Jr, Nam B, Bennett J, Masters G, Guarino M, Kumar A, Nefedova Y, Vonderheide RH, Languino LR, Abrams SI, Gabrilovich DI. Identification of monocyte-like precursors of granulocytes in cancer as a mechanism for accumulation of PMN-MDSCs[J]. J Exp Med, 2019, 216(9): 2150-2169.
[DOI]
|
| [19] |
Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses[J]. Cancer Res, 2004, 64(16): 5839-5849.
[DOI]
|
| [20] |
Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME, Ochoa AC, Fletcher M, Velasco C, Wilk A, Reiss K, Rodriguez PC. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways[J]. Int J Cancer, 2014, 134(12): 2853-2864.
[DOI]
|
| [21] |
De Sanctis F, Sandri S, Ferrarini G, Pagliarello I, Sartoris S, Ugel S, Marigo I, Molon B, Bronte V. The emerging immunological role of post-translational modifications by reactive nitrogen species in cancer microenvironment[J]. Front Immunol, 2014, 5: 69.
[DOI]
|
| [22] |
Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond[J]. Nat Rev Drug Discov, 2019, 18(5): 379-401.
[DOI]
|
| [23] |
Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP, Laury-Kleintop LD, Mandik-Nayak L, Metz R, Ostrand-Rosenberg S, Prendergast GC, Muller AJ. IDO is a nodal pathogenic driver of lung cancer and metastasis development[J]. Cancer Discov, 2012, 2(8): 722-735.
[DOI]
|
| [24] |
Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T, Zou W, Rodriguez PC, Ochoa AC. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies[J]. Cancer Immunol Res, 2015, 3(11): 1236-1247.
[DOI]
|
| [25] |
Turbitt WJ, Collins SD, Meng H, Rogers CJ. Increased adiposity enhances the accumulation of MDSCs in the Tumor microenvironment and adipose tissue of pancreatic tumor-bearing mice and in immune organs of tumor-free hosts[J]. Nutrients, 2019, 11(12): 3012.
[DOI]
|
| [26] |
Patel S, Fu S, Mastio J, Dominguez GA, Purohit A, Kossenkov A, Lin C, Alicea-Torres K, Sehgal M, Nefedova Y, Zhou J, Languino LR, Clendenin C, Vonderheide RH, Mulligan C, Nam B, Hockstein N, Masters G, Guarino M, Schug ZT, Altieri DC, Gabrilovich DI. Unique pattern of neutrophil migration and function during tumor progression[J]. Nat Immunol, 2018, 19(11): 1236-1247.
[DOI]
|
| [27] |
LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology[J]. Nat Cell Biol, 2016, 18(4): 356-365.
[DOI]
|
| [28] |
Baumann T, Dunkel A, Schmid C, Schmitt S, Hiltensperger M, Lohr K, Laketa V, Donakonda S, Ahting U, Lorenz-Depiereux B, Heil JE, Schredelseker J, Simeoni L, Fecher C, Körber N, Bauer T, Hüser N, Hartmann D, Laschinger M, Eyerich K, Eyerich S, Anton M, Streeter M, Wang T, Schraven B, Spiegel D, Assaad F, Misgeld T, Zischka H, Murray PJ, Heine A, Heikenwälder M, Korn T, Dawid C, Hofmann T, Knolle PA, Höchst B. Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal[J]. Nat Immunol, 2020, 21(5): 555-566.
[DOI]
|
| [29] |
Fultang L, Panetti S, Ng M, Collins P, Graef S, Rizkalla N, Booth S, Lenton R, Noyvert B, Shannon-Lowe C, Middleton G, Mussai F, De Santo C. MDSC targeting with Gemtuzumab ozogamicin restores T cell immunity and immunotherapy against cancers[J]. EBioMedicine, 2019, 47: 235-246.
[DOI]
|
| [30] |
Lancet JE, Moseley AB, Coutre SE, DeAngelo DJ, Othus M, Tallman MS, Litzow MR, Komrokji RS, Erba HP, Appelbaum FR. A phase 2 study of ATRA, arsenic trioxide, and gemtuzumab ozogamicin in patients with high-risk APL (SWOG 0535)[J]. Blood Adv, 2020, 4(8): 1683-1689.
[DOI]
|
| [31] |
Condamine T, Kumar V, Ramachandran IR, Youn JI, Celis E, Finnberg N, El-Deiry WS, Winograd R, Vonderheide RH, English NR, Knight SC, Yagita H, McCaffrey JC, Antonia S, Hockstein N, Witt R, Masters G, Bauer T, Gabrilovich DI. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis[J]. J Clin Invest, 2014, 124(6): 2626-2639.
[DOI]
|
| [32] |
Dominguez G A, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, Forero A, Bendell J, Witt R, Hockstein N, Kumar P, Gabrilovich DI. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody[J]. Clin Cancer Res, 2017, 23(12): 2942-2950.
[DOI]
|
| [33] |
Tavazoie MF, Pollack I, Tanqueco R, Ostendorf BN, Reis BS, Gonsalves FC, Kurth I, Andreu-Agullo C, Derbyshire ML, Posada J, Takeda S, Tafreshian KN, Rowinsky E, Szarek M, Waltzman RJ, McMillan EA, Zhao C, Mita M, Mita A, Chmielowski B, Postow MA, Ribas A, Mucida D, Tavazoie SF. LXR/ApoE activation restricts innate immune suppression in cancer[J]. Cell, 2018, 172(4): 825-840.
[DOI]
|
| [34] |
Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, Horn LA, Palena C, Schlom J, Maeda DY, Zebala JA, Clavijo PE, Allen C. Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-cell immunotherapy in head and neck cancer models[J]. Clin Cancer Res, 2020, 26(6): 1420-1431.
[DOI]
|
| [35] |
Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, Eberlein C, Candido JB, Clarke M, Nixon C, Connelly J, Jamieson N, Carter CR, Balkwill F, Chang DK, Evans TRJ, Strathdee D, Biankin AV, Nibbs RJB, Barry ST, Sansom OJ, Morton JP. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma[J]. Cancer Cell, 2016, 29(6): 832-845.
[DOI]
|
| [36] |
He YM, Li X, Perego M, Nefedova Y, Kossenkov AV, Jensen EA, Kagan V, Liu YF, Fu SY, Ye QJ, Zhou YH, Wei L, Gabrilovich DI, Zhou J. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation[J]. Nat Med, 2018, 24(2): 224-231.
[DOI]
|
| [37] |
Darcy CJ, Minigo G, Piera KA, Davis JS, McNeil YR, Chen Y, Volkheimer AD, Weinberg JB, Anstey NM, Woodberry T. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients[J]. Crit Care, 2014, 18(4): R163.
[DOI]
|
| [38] |
Janols H, Bergenfelz C, Allaoui R, Larsson AM, Rydén L, Björnsson S, Janciauskiene S, Wullt M, Bredberg A, Leandersson K. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases[J]. J Leukoc Biol, 2014, 96(5): 685-693.
[DOI]
|
| [39] |
Uhel F, Azzaoui I, Grégoire M, Pangault C, Dulong J, Tadié JM, Gacouin A, Camus C, Cynober L, Fest T, Le Tulzo Y, Roussel M, Tarte K. Early expansion of circulating granulocytic myeloid-derived suppressor cells predicts development of nosocomial infections in patients with sepsis[J]. Am J Respir Crit Care Med, 2017, 196(3): 315-327.
[DOI]
|
| [40] |
Ruan WS, Feng MX, Xu J, Xu YG, Song CY, Lin LY, Li L, Lu YQ. Early activation of myeloid-derived suppressor cells participate in sepsis-induced immune suppression via PD-L1/PD-1 axis[J]. Front Immunol, 2020, 11: 1299.
[DOI]
|
| [41] |
Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress[J]. J Immunol, 2006, 176(4): 2085-2094.
[DOI]
|
| [42] |
Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Müller M, Blander JM, Tacke F, Trautwein C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function[J]. J Exp Med, 2010, 207(7): 1453-1464.
[DOI]
|
| [43] |
Poe SL, Arora M, Oriss TB, Yarlagadda M, Isse K, Khare A, Levy DE, Lee JS, Mallampalli RK, Chan YR, Ray A, Ray P. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia[J]. Mucosal Immunol, 2013, 6(1): 189-199.
[DOI]
|
| [44] |
Arocena AR, Onofrio LI, Pellegrini AV, Carrera Silva AE, Paroli A, Cano RC, Aoki MP, Gea S. Myeloid-derived suppressor cells are key players in the resolution of inflammation during a model of acute infection[J]. Eur J Immunol, 2014, 44(1): 184-194.
[DOI]
|
| [45] |
Sanmarco LM, Visconti LM, Eberhardt N, Ramello MC, Ponce NE, Spitale NB, Vozza ML, Bernardi GA, Gea S, Minguez AR, Aoki MP. IL-6 Improves the nitric oxide-induced cytotoxic CD8+ T cell dysfunction in human chagas disease[J]. Front Immunol, 2016, 7: 626.
[DOI]
|
| [46] |
Davids M, Pooran A, Smith L, Tomasicchio M, Dheda K. The frequency and effect of granulocytic myeloid-derived suppressor cells on mycobacterial survival in patients with tuberculosis: a preliminary report[J]. Front Immunol, 2021, 12: 676679.
[DOI]
|
| [47] |
Grassi G, Vanini V, De Santis F, Romagnoli A, Aiello A, Casetti R, Cimini E, Bordoni V, Notari S, Cuzzi G, Mosti S, Gualano G, Palmieri F, Fraziano M, Goletti D, Agrati C, Sacchi A. PMN-MDSC frequency discriminates active versus latent tuberculosis and could play a role in counteracting the immune-mediated lung damage in active disease[J]. Front Immunol, 2021, 12: 594376.
[DOI]
|
| [48] |
Parveen S, Lun S, Urbanowski ME, Cardin M, Shen J, Murphy JR, Bishai WR. Effective host-directed therapy for tuberculosis by targeted depletion of myeloid-derived suppressor cells and related cells using a diphtheria toxin-based fusion protein[J]. J Infect Dis, 2021, 224(11): 1962-1972.
[DOI]
|
| [49] |
Jøntvedt Jørgensen M, Jenum S, Tonby K, Mortensen R, Walzl G, Du Plessis N, Dyrhol-Riise AM. Monocytic myeloid-derived suppressor cells reflect tuberculosis severity and are influenced by cyclooxygenase-2 inhibitors[J]. J Leukoc Biol, 2021, 110(1): 177-186.
[DOI]
|
| [50] |
Leukes VN, Dorhoi A, Malherbe ST, Maasdorp E, Khoury J, McAnda S, Walzl G, du Plessis N. Targeting of myeloid-derived suppressor cells by all-trans retinoic acid as host-directed therapy for human tuberculosis[J]. Cell Immunol, 2021, 364: 104359.
[DOI]
|
| [51] |
Gupta S, Krug S, Pokkali S, Leanderson T, Isaacs JT, Srikrishna G, Bishai WR. Pharmacologic exhaustion of suppressor cells with tasquinimod enhances bacterial clearance during tuberculosis[J]. Am J Respir Crit Care Med, 2019, 199(3): 386-389.
[DOI]
|
| [52] |
Zhang ZN, Yi N, Zhang TW, Zhang LL, Wu X, Liu M, Fu YJ, He SJ, Jiang YJ, Ding HB, Chu ZX, Shang H. Myeloid-derived suppressor cells associated with disease progression in primary HIV infection: PD-L1 blockade attenuates inhibition[J]. J Acquir Immune Defic Syndr, 2017, 76(2): 200-208.
[DOI]
|
| [53] |
Tumino N, Turchi F, Meschi S, Lalle E, Bordoni V, Casetti R, Agrati C, Cimini E, Montesano C, Colizzi V, Martini F, Sacchi A. In HIV-positive patients, myeloid-derived suppressor cells induce T-cell anergy by suppressing CD3ζ expression through ELF-1 inhibition[J]. AIDS, 2015, 29(18): 2397-2407.
[DOI]
|
| [54] |
Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, Liu Y, Yan D, Hu F, Guo P, Chen X, Chen L, Zhang H, Tang X, Zhou J. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals[J]. J Virol, 2013, 87(3): 1477-1490.
[DOI]
|
| [55] |
Collins PJ, Fox CP, George L, Pearce H, Ryan G, De Santo C, Mussai F, Lewis D, Long H, Shannon-Lowe C. Characterizing EBV-associated lymphoproliferative diseases and the role of myeloid-derived suppressor cells[J]. Blood, 2021, 137(2): 203-215.
[DOI]
|
| [56] |
Sinha D, Khanna R. EBV and myeloid-derived suppressor cells[J]. Blood, 2021, 137(2): 148-150.
[DOI]
|
| [57] |
Norris BA, Uebelhoer LS, Nakaya HI, Price AA, Grakoui A, Pulendran B. Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity[J]. Immunity, 2013, 38(2): 309-321.
[DOI]
|
| [58] |
Taleb K, Auffray C, Villefroy P, Pereira A, Hosmalin A, Gaudry M, Le Bon A. Chronic type Ⅰ IFN is sufficient to promote immunosuppression through accumulation of myeloid-derived suppressor cells[J]. J Immunol, 2017, 198(3): 1156-1163.
[DOI]
|
| [59] |
Ren JP, Zhao J, Dai J, Griffin JW, Wang L, Wu XY, Morrison ZD, Li GY, El Gazzar M, Ning SB, Moorman JP, Yao ZQ. Hepatitis C virus-induced myeloid-derived suppressor cells regulate T-cell differentiation and function via the signal transducer and activator of transcription 3 pathway[J]. Immunology, 2016, 148(4): 377-386.
[DOI]
|
| [60] |
Telatin V, Nicoli F, Frasson C, Menegotto N, Barbaro F, Castelli E, Erne E, Palù G, Caputo A. In chronic hepatitis C infection, myeloid-derived suppressor cell accumulation and T cell dysfunctions revert partially and late after successful direct-acting antiviral treatment[J]. Front Cell Infect Microbiol, 2019, 9: 190.
[DOI]
|
| [61] |
Huang A, Zhang B, Yan W, Wang B, Wei H, Zhang F, Wu L, Fan K, Guo Y. Myeloid-derived suppressor cells regulate immune response in patients with chronic hepatitis B virus infection through PD-1-induced IL-10[J]. J Immunol, 2014, 193(11): 5461-5469.
[DOI]
|
| [62] |
Fang Z, Li J, Yu X, Zhang D, Ren G, Shi B, Wang C, Kosinska AD, Wang S, Zhou X, Kozlowski M, Hu Y, Yuan Z. Polarization of monocytic myeloid-derived suppressor cells by hepatitis B surface antigen is mediated via ERK/IL-6/STAT3 signaling feedback and restrains the activation of T cells in chronic hepatitis B virus infection[J]. J Immunol, 2015, 195(10): 4873-4883.
[DOI]
|
| [63] |
Yang F, Yu X, Zhou C, Mao R, Zhu M, Zhu H, Ma Z, Mitra B, Zhao G, Huang Y, Guo H, Wang B, Zhang J. Hepatitis B e antigen induces the expansion of monocytic myeloid-derived suppressor cells to dampen T-cell function in chronic hepatitis B virus infection[J]. PLoS Pathog, 2019, 15(4): e1007690.
[DOI]
|
| [64] |
Pallett LJ, Gill US, Quaglia A, Sinclair LV, Jover-Cobos M, Schurich A, Singh KP, Thomas N, Das A, Chen A, Fusai G, Bertoletti A, Cantrell DA, Kennedy PT, Davies NA, Haniffa M, Maini MK. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells[J]. Nat Med, 2015, 21(6): 591-600.
[DOI]
|
| [65] |
Nan J, Xing YF, Hu B, Tang JX, Dong HM, He YM, Ruan DY, Ye QJ, Cai JR, Ma XK, Chen J, Cai XR, Lin ZX, Wu XY, Li X. Endoplasmic reticulum stress induced LOX-1(+) CD15(+) polymorphonuclear myeloid-derived suppressor cells in hepatocellular carcinoma[J]. Immunology, 2018, 154(1): 144-155.
[DOI]
|
| [66] |
Li T, Zhang X, Lv Z, Gao L, Yan H. Increased expression of myeloid-derived suppressor cells in patients with HBV-related hepatocellular carcinoma[J]. Biomed Res Int, 2020, 2020: 6527192.
[DOI]
|
| [67] |
Zeng Y, Li Y, Xu Z, Gan W, Lu L, Huang X, Lin C. Myeloid-derived suppressor cells expansion is closely associated with disease severity and progression in HBV-related acute-on-chronic liver failure[J]. J Med Virol, 2019, 91(8): 1510-1518.
[DOI]
|
| [68] |
Yu Z, Li J, Ren Z, Sun R, Zhou Y, Zhang Q, Wang Q, Cui G, Li J, Li A, Duan Z, Xu Y, Wang Z, Yin P, Piao H, Lv J, Liu X, Wang Y, Fang M, Zhuang Z, Xu G, Kan Q. Switching from fatty acid oxidation to glycolysis improves the outcome of acute-on-chronic liver failure[J]. Adv Sci (Weinh), 2020, 7(7): 1902996.
[DOI]
|
 2022, Vol. 17
2022, Vol. 17


