2. 浙江中医药大学医学技术与信息工程学院,浙江 杭州 310053
2. School of Medical Technology and Information Engineering, Zhejiang Chinese Medical University, Hangzhou 310053, Zhejiang Province, China
血流感染(bloodstream infection, BSI)是指病原微生物进入血液循环并生长繁殖,产生毒素、代谢产物,引发全身炎症反应的一类感染性疾病[1],临床常引起菌血症、败血症和脓毒症等。常见危险因素包括血管内导管的长期使用以及呼吸道感染、泌尿道感染、手术部位感染等[2-3]。近年来,随着人口老龄化的加剧和广谱抗生素的广泛使用,BSI的发病率及病死率呈逐年上升趋势[3]。
研究表明,BSI的主要病原微生物包括细菌和真菌,综合性医院与中医院的BSI感染情况以及不同地区来源的BSI病原菌种类及耐药性,均存在显著差异[4-5]。本研究对浙江省县级中医院新昌县中医院2019年1月—2023年6月的血培养样本进行回顾性分析,研究血培养阳性样本分离的病原菌分布及其药物敏感性情况,旨在为BSI的诊断和治疗提供依据。
1 材料和方法 1.1 研究对象收集2019年1月—2023年6月新昌县中医院送检的血培养样本。
1.2 主要仪器与试剂主要仪器为血培养仪(BACT/ALERT 3D,法国)、VITEK2全自动微生物生化鉴定仪及药敏分析系统(BACT/ALERT 3D,法国)、生化恒温培养箱(SPX-250B-Z型,上海)、霉菌恒温培养箱(MJX-160B-Z,上海)、生物安全柜(BSC-1500Ⅱ)。主要试剂为仪器配套血培养瓶、细菌鉴定卡及药敏卡,血琼脂平板、巧克力平板、麦康凯平板和厌氧专用血琼脂平板,均购自郑州安图生物公司,GENbag厌氧产气袋由法国梅里埃公司生产。
1.3 病原菌分离及鉴定将血培养瓶置于血液培养仪中振荡培养,若血液培养仪报告为阳性,将其转种至相应培养基中,采用梅里埃VITEK2全自动微生物鉴定仪进行菌种鉴定。
1.4 药物敏感试验使用梅里埃VITEK-2药敏分析系统进行药敏试验,结果参照美国临床实验室标准化委员会(Clinical and Laboratory Standards Institute,CLSI,2019)标准进行结果判读[6]。质控菌株为大肠埃希菌ATCC 25922、铜绿假单胞菌ATCC 27853、金黄色葡萄球菌ATCC25923。
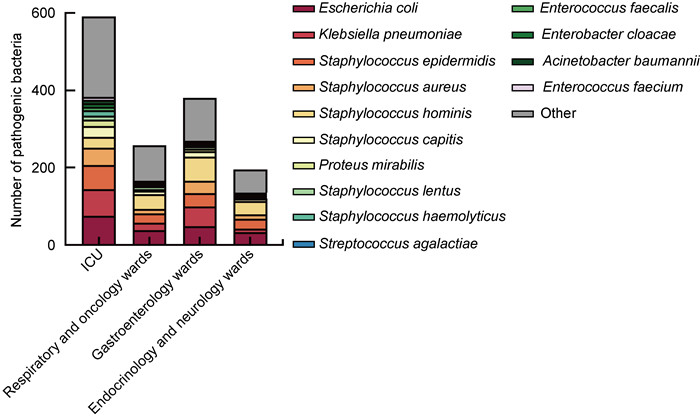
2 结果 2.1 病区及菌群分布1 692例血培养阳性标本主要来自重症监护室(34.87%)、呼吸科与肿瘤科病区(22.46%)、消化病区(15.25%)和内分泌科与神经内科病区(11.58%)等(见表 1)。阳性标本分离最多的前4病区病原菌的具体分布如图 1所示,大肠埃希菌的分离率最高,其次是肺炎克雷伯菌、表皮葡萄球菌和金黄色葡萄球菌。
| Ward | Positive specimen (n) | Composition ratio (%) |
| Intensive care unit (ICU) | 590 | 34.87 |
| Respiratory and oncology wards | 380 | 22.46 |
| Gastroenterology ward | 258 | 15.25 |
| Endocrinology and neurology wards | 196 | 11.58 |
| Cardiology ward | 70 | 4.14 |
| Neurosurgery and urology wards | 55 | 3.25 |
| Pediatric ward | 39 | 2.30 |
| General surgery ward | 31 | 1.83 |
| Orthopedics ward | 30 | 1.77 |
| Obstetrics and gynecology ward | 28 | 1.65 |
| General medicine ward | 15 | 0.89 |
| Total | 1 692 | 100.00 |
| Note: The respiratory and oncology departments are merged into one ward, the endocrinology and neurology departments are merged into one ward, the neurosurgery and urology departments are merged into one ward. | ||
|
| 图 1 分离率居前4位的病区中血流感染病原菌构成情况 Fig. 1 The composition of pathogenic microorganisms causing bloodstream infections in the top 4 wards of separation rate |
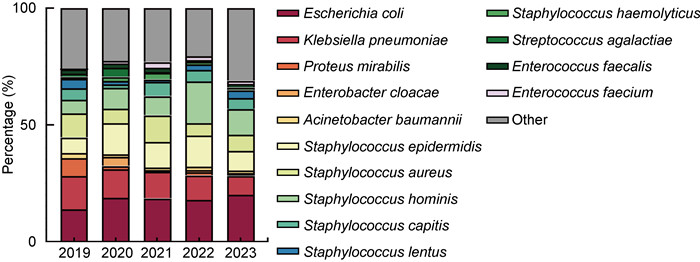
1 692例血培养阳性标本,其中需氧菌1 637株(96.74%),厌氧菌19株(1.12%),真菌36株(2.13%);革兰氏阴性菌主要为大肠埃希菌、肺炎克雷伯菌,革兰氏阳性菌主要为人葡萄球菌、表皮葡萄球菌和金黄色葡萄球菌,具体如表 2所示。5年间,大肠埃希菌和人葡萄球菌的检出率逐年增高,肺炎克雷伯菌的检出率逐渐降低(见图 2)。
| Bacterium | Amount | Component ratio (%) |
| Gram-negative bacterium | ||
| Escherichia coli | 267 | 15.78 |
| Klebsiella pneumoniae | 167 | 9.87 |
| Proteus mirabilis | 29 | 1.71 |
| Enterobacter cloacae | 22 | 1.30 |
| Acinetobacter baumannii | 20 | 1.18 |
| Serratia marcescens | 6 | 0.35 |
| Other gram-negative bacterium | 58 | 3.43 |
| Gram-positive bacterium | ||
| Staphylococcus hominis | 178 | 10.52 |
| Staphylococcus epidermidis | 164 | 9.69 |
| Staphylococcus aureus | 115 | 6.80 |
| Staphylococcus capitis | 65 | 3.84 |
| Staphylococcus lentus | 35 | 2.07 |
| Staphylococcus haemolyticus | 24 | 1.42 |
| Streptococcus agalactiae | 18 | 1.06 |
| Klebsiella | 18 | 1.06 |
| Escherichia coli | 22 | 1.30 |
| Streptococcus penumoniae | 27 | 1.60 |
| Other gram-positive bacterium | 402 | 23.76 |
| Fungus | ||
| Candida glabrata | 9 | 0.53 |
| Candida albicans | 6 | 0.35 |
| Candida krusei | 3 | 0.18 |
| Candida tropicalis | 4 | 0.24 |
| Candida parapsilosis | 4 | 0.24 |
| Candida spp. | 10 | 0.59 |
| Anaerobic bacteria* | 19 | 1.12 |
| Total | 1 692 | 100.00 |
| *Strains of anaerobic bacteria were not identified. | ||
|
| 图 2 血培养阳性样本病原菌5年分布变化 Fig. 2 Changes in the distribution of pathogenic bacteria in blood culture positive samples over five years |
对上述分离的病原菌进行药敏试验,结果显示,革兰氏阴性菌和革兰氏阳性菌对临床常用抗生素均有较高的敏感性。革兰氏阴性菌中,大肠埃希菌和肺炎克雷伯菌对头孢类、氨曲南、丁胺卡那、厄他培南和哌拉西林/他唑巴坦等抗菌药物的敏感率超过70%,但对氨苄西林的耐药率较高(>80%);而奇异变形杆菌对氨苄西林、氨苄西林/舒巴坦、复方新诺明、呋喃妥因的耐药率超过70%(见表 3)。革兰氏阳性菌中,位列前3位的人葡萄球菌、表皮葡萄球菌和金黄色葡萄球菌对利奈唑烷、替加环素、呋喃妥因和喹呶普汀/达福普汀等抗菌药物的敏感率达90%以上,且未发现万古霉素耐药菌株(见表 4)。
| Antibiotics | Escherichia coli (n=276) | Klebsiella pneumoniae (n=167) | Proteus mirabilis (n=29) | |||||||||
| Sensitive (%) | Intermediate (%) | Resistant (%) | Sensitive (%) | Intermediate (%) | Resistant (%) | Sensitive (%) | Intermediate (%) | Resistant (%) | ||||
| Beta-lactams | Amoxicillin | 198 (74.16) | 4 (1.50) | 65 (24.34) | 140 (83.83) | 0 (0.00) | 27 (16.17) | 29 (100.00) | 0 (0.00) | 0 (0.00) | ||
| Ampicillin | 51 (19.10) | 2 (0.75) | 214 (80.15) | 0 (0.00) | 0 (0.00) | 167 (100.00) | 5 (17.24) | 0 (0.00) | 24 (82.76) | |||
| Ertapenem* | 267 (100.00) | 0 (0.00) | 0 (0.00) | 151 (100.00) | 0 (0.00) | 0 (0.00) | 27 (100.00) | 0 (0.00) | 0 (0.00) | |||
| Ceftriaxone | 155 (58.05) | 10 (3.75) | 102 (38.20) | 137 (82.04) | 0 (0.00) | 30 (17.96) | 25 (86.21) | 4 (13.79) | 0 (0.00) | |||
| Cefotaxime | 209 (78.28) | 0 (0.00) | 58 (21.72) | 140 (83.83) | 1 (0.60) | 26 (15.57) | 24 (82.76) | 1 (3.45) | 4 (13.79) | |||
| Ceftazidime | 259 (97.00) | 6 (2.25) | 2 (0.75) | 151 (90.42) | 0 (0.00) | 16 (9.58) | 29 (100.00) | 0 (0.00) | 0 (0.00) | |||
| Cefepime* | 206 (82.07) | 3 (1.20) | 42 (16.73) | 143 (86.67) | 0 (0.00) | 22 (13.33) | 29 (100.00) | 0 (0.00) | 0 (0.00) | |||
| Cefuroxime* | 117 (54.17) | 4 (1.85) | 95 (43.98) | 120 (75.47) | 9 (5.66) | 30 (18.87) | 0 (0.00) | 5 (21.74) | 18 (78.26) | |||
| Imipenem | 265 (99.25) | 2 (0.75) | 0 (0.00) | 143 (85.63) | 2 (1.20) | 22 (13.17) | / | / | / | |||
| Piperacillin/Tazobactam | 254 (95.13) | 7 (2.62) | 6 (2.25) | 145 (86.83) | 2 (1.20) | 20 (11.98) | 29 (100.00) | 0 (0.00) | 0 (0.00) | |||
| Ampicillin/Sulbactam | 82 (30.71) | 61 (22.85) | 124 (46.44) | 131 (78.44) | 1 (0.60) | 35 (20.96) | 5 (17.24) | 0 (0.00) | 24 (82.76) | |||
| Aminoglycosides | Gentamicin* | 208 (96.30) | 0 (0.00) | 8 (3.70) | 149 (93.71) | 0 (0.00) | 10 (6.29) | 23 (100.00) | 0 (0.00) | 0 (0.00) | ||
| Vancomycin | 185 (69.29) | 0 (0.00) | 82 (30.71) | 153 (91.62) | 0 (0.00) | 14 (8.38) | 7 (24.14) | 16 (55.17) | 6 (20.69) | |||
| Tobramycin | 182 (68.16) | 52 (19.48) | 33 (12.36) | 145 (86.83) | 12 (7.19) | 10 (5.99) | 11 (37.93) | 14 (48.28) | 4 (13.79) | |||
| Quinolones | Ciprofloxacin | 112 (41.95) | 30 (11.24) | 125 (46.82) | 114 (68.26) | 12 (7.19) | 41 (24.55) | 11 (37.93) | 2 (6.90) | 16 (55.17) | ||
| Levofloxacin | 45 (16.85) | 113 (42.32) | 109 (40.82) | 114 (68.26) | 26 (15.57) | 27 (16.17) | 8 (27.59) | 5 (17.24) | 16 (55.17) | |||
| Nitrofurans | Furazolidone | 261 (97.75) | 5 (1.87) | 1 (0.37) | 51 (30.54) | 85 (50.90) | 31 (18.56) | 0 (0.00) | 0 (0.00) | 29 (100.00) | ||
| Sulfonamides | Compound neomycin | 141 (52.81) | 0 (0.00) | 126 (47.19) | 130 (77.84) | 0 (0.00) | 37 (22.16) | 5 (17.24) | 0 (0.00) | 24 (82.76) | ||
| *: Partial Klebsiella pneumoniae isolates did not undergo sensitivity testing for ertapenem, cefepime, cefuroxime, and gentamicin. /: For Proteus mirabilis, imipenem sensitivity testing was not conducted. |
||||||||||||
| Antibiotics | Staphylococcus saprophyticus (n=178) | Staphylococcus epidermidis (n=162) | Staphylococcus aureus (n=115) | |||||||||
| Sensitive (%) | Intermediate (%) | Resistant (%) | Sensitive (%) | Intermediate (%) | Resistant (%) | Sensitive (%) | Intermediate (%) | Resistant (%) | ||||
| Beta-lactams | Benzylpenicillin | 80 (44.94) | 0 (0.00) | 98 (55.06) | 37 (22.84) | 0 (0.00) | 125 (77.16) | 105 (91.30) | 0 (0.00) | 10 (8.70) | ||
| Penicillin | 40 (22.47) | 0 (0.00) | 138 (77.53) | 8 (4.94) | 0 (0.00) | 154 (95.06) | 11 (9.57) | 0 (0.00) | 104 (90.43) | |||
| Quinolones | Ciprofloxacin | 115 (64.61) | 13 (7.30) | 50 (28.09) | 74 (45.68) | 31 (19.14) | 57 (35.19) | 78 (67.83) | 5 (4.35) | 32 (27.83) | ||
| Moxifloxacin | 124 (69.66) | 18 (10.11) | 36 (20.22) | 74 (45.68) | 76 (46.91) | 12 (7.41) | 81 (70.43) | 15 (13.04) | 19 (16.52) | |||
| Levofloxacin | 120 (67.42) | 20 (11.24) | 38 (21.35) | 74 (45.68) | 3 (1.85) | 85 (52.47) | 81 (70.43) | 0 (0.00) | 34 (29.57) | |||
| Sulfonamides | Compound sulfamethoxazole | 136 (76.40) | 0 (0.00) | 42 (23.60) | 60 (37.04) | 0 (0.00) | 102 (62.96) | 103 (89.57) | 0 (0.00) | 12 (10.43) | ||
| Macrolides | Erythromycin | 41 (23.03) | 1 (0.56) | 136 (76.40) | 63 (38.89) | 0 (0.00) | 99 (61.11) | 66 (57.39) | 0 (0.00) | 49 (42.61) | ||
| Lincomycins | Clindamycin | 93 (52.25) | 2 (1.12) | 83 (46.63) | 90 (55.56) | 0 (0.00) | 72 (44.44) | 71 (61.7) | 0 (0.00) | 44 (38.26) | ||
| Rifamycins | Rifampicin | 170 (95.51) | 1 (0.56) | 7 (3.93) | 159 (98.15) | 0 (0.00) | 3 (1.85) | 115 (100.00) | 0 (0.00) | 0 (0.00) | ||
| Oxazolidinones | Linezolid | 178 (100.00) | 0 (0.00) | 0 (0.00) | 162 (100.00) | 0 (0.00) | 0 (0.00) | 115 (100.00) | 0 (0.00) | 0 (0.00) | ||
| Aminoglycosides | Gentamicin | 172 (96.63) | 5 (2.81) | 1 (0.56) | 146 (90.12) | 6 (3.70) | 10 (6.17) | 114 (99.13) | 0 (0.00) | 1 (0.87) | ||
| Tetracyclines | Tetracycline | 137 (76.97) | 1 (0.56) | 40 (22.47) | 149 (91.98) | 1 (0.62) | 12 (7.41) | 87 (75.65) | 0 (0.00) | 28 (24.35) | ||
| Glycylcyclines | Tigecycline | 178 (100.00) | 0 (0.00) | 0 (0.00) | 162 (100.00) | 0 (0.00) | 0 (0.00) | 115 (100.00) | 0 (0.00) | 0 (0.00) | ||
| Glycopeptides | Vancomycin | 178 (100.00) | 0 (0.00) | 0 (0.00) | 161 (99.38) | 1 (0.62) | 0 (0.00) | 113 (98.26) | 2 (1.74) | 0 (0.00) | ||
| Nitrofurans | Furazolidone | 175 (98.31) | 2 (1.12) | 1 (0.56) | 162 (100.00) | 0 (0.00) | 0 (0.00) | 115 (100.00) | 0 (0.00) | 0 (0.00) | ||
| Glycylglycines | Quinupristin/Dalfopristin | 176 (98.88) | 1 (0.56) | 1 (0.56) | 162 (100.00) | 0 (0.00) | 0 (0.00) | 115 (100.00) | 0 (0.00) | 0 (0.00) | ||
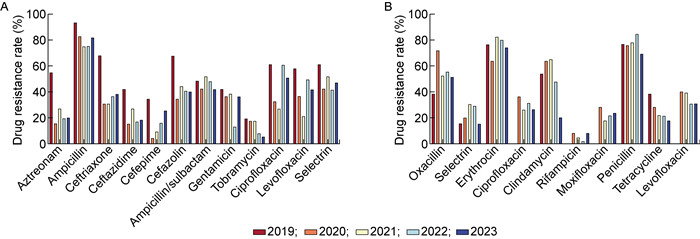
本研究分析了大肠埃希菌和人葡萄球菌5年耐药率的变化情况,如图 3所示。大肠埃希菌在5年间对妥布霉素的耐药率呈下降趋势,而在2020—2023年对头孢曲松、头孢吡肟等抗菌药物的耐药性呈上升趋势。人葡萄球菌在5年间对四环素的耐药率呈下降趋势,在2021—2023年对莫西沙星的耐药率呈上升趋势。
|
| A: 大肠埃希菌;B: 人葡萄球菌。 A: E. coli; B: S. hominis. 图 3 近5年大肠埃希菌和人葡萄球菌耐药率变化 Fig. 3 Changes of drug resistance rates of E. coli and S. hominis in recent five years |
BSI是危重患者常见且危及生命的并发症,通常会导致感染性休克和死亡[7]。中医对血流感染的辨证有多种不同的方法,有利于临床辨证诊断,提高相关血液疾病的临床疗效[8]。本研究发现,与综合医院相比,中医院血流感染的革兰氏阳性菌比例(63.12%)显著较高[9]。但病原菌分布与综合医院和其他中医院类似,三甲综合医院和三甲中医院血液样本中分离得到的革兰氏阴性菌主要为大肠埃希菌和肺炎克雷伯菌,革兰氏阳性菌主要为凝固酶阴性葡萄球菌和金黄色葡萄球菌[9-11],与本研究结果基本一致。
本研究共收集1 692例血流感染阳性样本,主要分离自重症监护室、肿瘤科等患者免疫力低下的病区,而三甲综合医院血流感染主要来自血液内科、老年病科、重症监护室等病区[10-13]。病原菌的药物敏感性也存在一定差异,可能与地区差异、医院患者类型、临床治疗方式等因素相关。大肠埃希菌对头孢曲松和头孢吡肟等抗菌药物的耐药性呈上升趋势,临床应根据药敏结果选择合适的抗菌药物,以提高治疗效率。
血流感染疾病的临床检测与诊断技术在不断发展。血培养是目前血流感染诊断的金标准[14],但其检出率低、检测周期相对长(一般5~7天),可能延误诊断,进而影响患者预后[15]。因此,需要快速可靠的新技术,以提高血培养检测效率。目前,在临床应用的高通量测序技术(next-generation sequencing,NGS),可以对血液样本中所有的DNA和RNA进行测序,然后对每个序列进行精确的分类与鉴定[16]。利用基质辅助激光解吸电离飞行时间质谱(matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, MALDI-TOF MS),可在5分钟内得到鉴定结果,并可直接鉴定阳性血培养瓶中的病原菌[17]。
综上所述,临床应积极运用新的快速可靠的诊断工具,来提高血培养的检测效率。临床应加强血流感染病原菌的检测,并分析病原菌的药物敏感性情况,以提高血流感染的诊断效率和治疗效果。
| [1] |
金君, 孙仁华, 呼邦传. 血流感染的分子诊断研究进展[J]. 中国现代医生, 2020, 58(36): 182-187. [URI]
|
| [2] |
Mathur P, Varghese P, Tak V, Gunjiyal J, Lalwani S, Kumar S, Misra MC. Epidemiology of blood stream infections at a level-1 trauma care center of India[J]. J Lab Physicians, 2014, 6(1): 22-27.
[DOI]
|
| [3] |
Taylor G, Buchanan-Chell M, Kirkland T, McKenzie M, Wiens R. Long term trends in the occurrence of nosocomial blood stream infection[J]. Can J Infect Dis, 2000, 11(1): 29-33.
[DOI]
|
| [4] |
周梦兰, 杨启文, 于淑颖, 徐英春. 血流感染流行病学研究进展[J]. 中国感染与化疗杂志, 2019, 19(02): 212-217. [DOI]
|
| [5] |
王妍妍, 蒋昭清, 冯丹丹, 干铁儿, 阮云双, 吴建浓. 医院获得性革兰氏阴性杆菌血流感染的耐药性及预后影响因素分析[J]. 浙江临床医学, 2022, 24(2): 216-219. [URI]
|
| [6] |
Khan MS, Kareem A, Fatima K, Rauf S, Khalid A, Bashir MS. Microbial patterns and antibiotic susceptibility in blood culture isolates of septicemia suspected children in the pediatrics ward of a tertiary care hospital[J]. J Lab Physicians, 2021, 13(1): 64-69.
[DOI]
|
| [7] |
Hu B, Tao Y, Shao Z, Zheng Y, Zhang R, Yang X, Liu J, Li X, Sun R. A comparison of blood pathogen detection among droplet digital PCR, metagenomic next-generation sequencing, and blood culture in critically ill patients with suspected bloodstream infections[J]. Front Microbiol, 2021, 12: 641202.
[DOI]
|
| [8] |
刘凤霞, 张弘. 血液病的中医辨证研究进展[J]. 中国医药指南, 2011, 9(35): 299-301. [DOI]
|
| [9] |
赵妍, 杨柳, 王小琴. 中医院血液科血流感染病原菌分布及耐药分析[J]. 重庆医学, 2020, 49(06): 905-909. [DOI]
|
| [10] |
张盼, 谢守军, 温海楠. 1113株血流感染分离病原菌状况及耐药性特征[J]. 临床荟萃, 2019, 34(02): 148-153. [DOI]
|
| [11] |
钟一梅, 李月桂, 谭晓宇, 黄晨娟. 广东省江门市某三甲中医院2016—2021年血流感染主要病原菌及抗菌药物耐药性分析[J]. 国外医药(抗生素分册), 2023, 44(4): 246-251. [DOI]
|
| [12] |
胡志清, 徐翠红, 刘坦. 消化系统恶性肿瘤患者化疗后血流感染病原菌及其耐药性分析[J]. 中国卫生检验杂志, 2022, 32(5): 544-547. [URI]
|
| [13] |
杨玉芳, 赵群, 蔡继明, 沈杰, 吴晓燕, 王庆宇, 徐少毅. 综合ICU患者血流感染病原菌分布与耐药性的调查分析[J]. 浙江临床医学, 2021, 23(9): 1295-1297. [URI]
|
| [14] |
Martinez RM, Wolk DM. Bloodstream infections[J]. Microbiol Spectr, 2016, 4(4).
[DOI]
|
| [15] |
Lamy B, Sundqvist M, Idelevich EA, ESCMID Study Group for Bloodstream Infections, Endocarditis and Sepsis (ESGBIES). Bloodstream infections-Standard and progress in pathogen diagnostics[J]. Clin Microbiol Infect, 2020, 26(2): 142-150.
[DOI]
|
| [16] |
Greninger AL, Naccache SN. Metagenomics to assist in the diagnosis of bloodstream infection[J]. J Appl Lab Med, 2019, 3(4): 643-653.
[DOI]
|
| [17] |
Clerc O, Prod'hom G, Vogne C, Bizzini A, Calandra T, Greub G. Impact of matrix-assisted laser desorption ionization time-of-flight mass spectrometry on the clinical management of patients with Gram-negative bacteremia: a prospective observational study[J]. Clin Infect Dis, 2013, 56(8): 1101-1107.
[DOI]
|
 2024, Vol. 19
2024, Vol. 19


