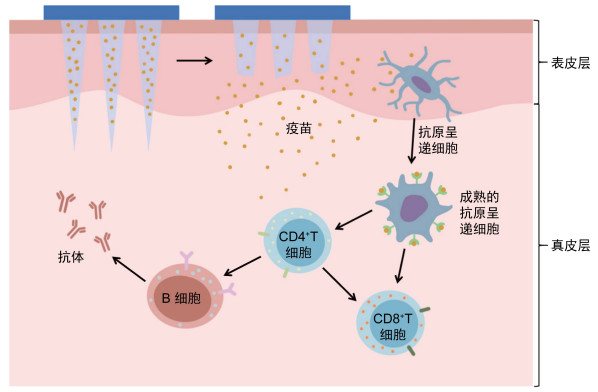
使用微针阵列进行皮下疫苗注射是疫苗接种的一个新思路,通过皮下注射疫苗可以使皮肤表皮层和真皮层内丰富的抗原呈递细胞(antigen-presenting cells,APC)参与抗原的摄取与提呈[1]。APC摄取抗原并加工后,将其提呈给淋巴细胞,通过体液免疫和细胞免疫途径对进入机体的抗原产生免疫应答(见图 1)。微针种类包括固体微针、涂层微针、空心微针、可溶性微针(dissolving microneedles, dMNs)和水凝胶微针[2-4]。其中dMNs是一种将疫苗溶液制成固体针尖刺入真皮层,通过针尖的溶解释放抗原从而达到免疫效果的微针类型,具有无痛[5]、微创[6]、操作便捷[7]、载药量大、不产生针尖废弃物[8]等诸多优点。dMNs优势明显,有广泛的临床应用前景,因此探究dMNs经皮免疫的应答过程与疫苗的免疫效果能够为此类疫苗研发奠定基础。本文分别从体液免疫应答和细胞免疫应答2个角度总结了目前dMNs疫苗的相关研究进展,以期为dMNs的广泛应用提供参考。
|
| 图 1 可溶性微针疫苗激活免疫反应 Fig. 1 Immune response activated by dissolving microneedle vaccine |
应用dMNs经皮免疫可诱导有效的体液免疫应答。研究表明,dMNs能有效诱导特异性抗体的产生[9],且可以达到与肌肉注射组相当或者更高水平的抗体滴度[10-12];不仅如此,Raphael等[13]对负载流感疫苗dMNs的研究表明,在免疫后第28天,0.12 μg dMNs组的免疫球蛋白G(immunoglobulin G,IgG)水平与5 μg肌肉注射组相当,并且0.12 μg dMNs组在免疫102天后仍可保持较强的免疫应答,说明使用低剂量的dMNs疫苗即可达到良好的免疫效果,可节约疫苗的抗原用量。同样,针对轮状病毒、脊髓灰质炎的dMNs疫苗研究亦有类似的发现,因此dMNs是一种经济的疫苗制备手段[9, 14]。
1.2 增加分泌IL-4因子的脾细胞数量脾细胞的细胞因子分泌水平是反映抗原特异性免疫应答强度的重要指标之一。白细胞介素4(interleukin-4,IL-4)可影响辅助性T细胞(helper T cell,Th)Th0细胞向Th2细胞分化,从而刺激B细胞增殖并产生抗体参与体液免疫。Tian等[11]的研究显示,用负载流感疫苗的dMNs免疫小鼠后,产生IL-4脾细胞的数量与肌肉注射组无明显差异,且两组最终产生的抗体滴度相当,以上结果说明dMNs疫苗可引起与肌肉注射相近的体液免疫应答效果。
2 dMNs疫苗可诱导有效的细胞免疫应答 2.1 增强抗原呈递能力树突状细胞是免疫应答过程中的专职APC,参与内源性与外源性抗原的呈递,从而启动针对特定抗原的适应性免疫应答。其中,内源性抗原被蛋白酶体降解为抗原肽后,在多种蛋白的作用下与主要组织相容性复合体(major histocompatibility complex,MHC)I类分子结合,最终提呈给CD8+T细胞。而外源性抗原则首先被树突状细胞摄取进入胞内形成内体,与溶酶体结合后被降解为抗原肽,随后MHC Ⅱ类分子进入内体与抗原肽结合,最终将其提呈给CD4+T细胞。
相较于肌肉注射将疫苗聚集于一点,dMNs含有数百个针头,因此当微针释放抗原至表皮层和真皮层时,可使抗原在皮肤内分布更均匀[15],从而被更多的树突状细胞摄取和提呈,并促进了树突状细胞的成熟和MHC表达的增加。Lee等[16]开发了一种副戈登分枝杆菌的dMNs(Mpg-MNP)疫苗,用于卡介苗的异源加强免疫,并观察到Mpg-MNP组树突状细胞表面的CD40、CD80、CD86及MHC II表达水平较皮下注射组有显著的上调,说明dMNs促进了树突状细胞的成熟,从而明显增强了卡介苗的免疫效果。
2.2 活化T细胞,增强细胞免疫应答高度成熟的树突状细胞可诱导T细胞活化,表达活化标记物干扰素γ(interferon-gamma,IFN-γ)和肿瘤坏死因子α (tumor necrosis factor-alpha,TNF-α),促进Th1型细胞免疫反应增强。一项对寨卡病毒灭活疫苗dMNs的研究发现,与肌肉注射组相比,dMNs疫苗接种可使小鼠淋巴结和脾中CD4+和CD8+T细胞数量增加并维持在一定水平,且分泌更高水平的IFN-γ和TNF-α [17]。另一项对结核疫苗dMNs的研究也显示出类似的结果[18],提示dMNs较于肌肉注射能诱导更强大的Th1型细胞免疫应答。一项针对装载新型冠状病毒DNA疫苗dMNs(MAP-1002)的研究显示,免疫后2~5周,小鼠引流淋巴结中细胞毒性T细胞(cytotoxic T lymphocyte,CTL)的水平上升,说明应用dMNs也可促进CTL的激活,以此杀伤靶细胞[19]。并且该病毒变异毒株的改进疫苗MAP-1016的研究结果也与之类似[20]。不仅如此,一项针对EV71病毒样颗粒的dMNs的研究报道,dMNs接种的剂量仅需肌肉注射组剂量的1/10,即可诱导更强的EV71特异性的T细胞应答[21]。
此外,特异性细胞免疫应答还在抗肿瘤过程中发挥着重要作用。CD8+T细胞被证明在抗肿瘤免疫中发挥了较强的作用。Lee等[22]以卵白蛋白(ovalbumin, OVA)作为模型抗原,经dMNs免疫小鼠后,可诱导OVA特异性CTL应答,并在移植EG7-OVA细胞的小鼠肿瘤模型中,显示了良好的预防和治疗作用。因此,目前dMNs也被用于肿瘤免疫疗法。
3 dMNs疫苗对Th1/Th2平衡的影响Th1和Th2细胞为Th细胞的2个亚群,Th1与细胞免疫相关,Th2则与体液免疫相关。免疫应答类型的不同可通过IgG亚型反映,一般通过检测IgG2a水平反映Th1应答,而通过IgG1水平反映Th2应答。dMNs可能影响疫苗诱导的免疫应答类型,改变原有的Th1/Th2平衡。一项同样以OVA为模型抗原的疫苗研究结果显示,dMNs组的IgG2a/IgG1>1,说明dMNs诱导的免疫应答以Th1为主[23]。而针对重组金黄色葡萄球菌肠毒素B蛋白的dMNs疫苗则主要诱导Th2型免疫应答[24]。Guo等[25]制作的一种由脂质体包裹OVA的dMNs则显示了相对平衡的Th1/Th2反应。综上所述,dMNs诱导Th1/Th2的应答类型可能受到抗原类型、疫苗浓度[18]、添加佐剂种类[26-27]以及佐剂与疫苗的比例等因素的影响。
4 dMNs疫苗优化策略为进一步增强dMNs的免疫效果,在dMNs制作过程中选择和使用不同种类的佐剂是一种有效的策略。佐剂能够增强疫苗的免疫原性,增强机体对抗原的特异性免疫应答。目前,主要应用的dMNs佐剂包括以下几类。
4.1 Toll样受体(toll-like receptors,TLR)激动剂TLR是参与固有免疫反应的一类在细胞表面或内吞体内表达的受体,它可以识别微生物分子并激活免疫细胞应答。同时,它也可以通过激活APC,成为连接固有免疫和特异性免疫的桥梁[28]。因此,将TLR激动剂作为佐剂加入疫苗中可以增强免疫应答[29]。一项针对猪生殖与呼吸综合征病毒的dMNs疫苗的研究结果显示,在免疫早期的引流淋巴结中,TLR7/8激动剂组可检测到APC活化或迁移相关基因CCR7表达水平上调,说明TLR7/8激动剂可诱导树突状细胞成熟,增强其抗原呈递能力[30]。目前,在哺乳动物中已发现11种TLRs,其中作为佐剂应用于疫苗的有TLR2、TLR3、TLR4、TLR5、TLR7/8、TLR9激动剂以及TLR联合激动剂,这些佐剂功能不同,且各有其优势与缺陷[31],因此对于TLR佐剂的安全性和有效性还需要更多研究结果的支持。
4.2 纳米颗粒纳米类佐剂是通过离子间的吸引与排斥实现抗原的递送,尤其有助于亲水性抗原的递送,同时保护抗原免于降解。常见的纳米类佐剂包括如下几类。聚乳酸-羟基乙酸共聚物[poly(lactic-co-glycolic acid),PLGA]纳米颗粒是一种常用的疫苗递送系统,目前已经被用于多种疾病疫苗的研究,如乙型肝炎[32],新型冠状病毒肺炎[33-34]和肿瘤[35]等。一项针对产气荚膜梭菌ε毒素dMNs的研究表明,PLGA纳米颗粒可以与APC相互作用以诱导良好的细胞及体液免疫应答,并可对小鼠提供100%保护[36]。此外,一些研究还使用含有聚肌胞[poly(I: C)]纳米复合物的dMNs,结果表明这种微针可以提高特异性抗体的水平[37],并增强CTL免疫[38]。壳聚糖纳米颗粒也是纳米佐剂的一种,已被应用于黑色素瘤的dMNs研究,该研究中佐剂与微针的联合使用,不仅增强了特异性抗体应答,而且促进了肿瘤的清除[39]。另一项加入壳寡糖的新型冠状病毒疫苗dMNs的研究检测到可同时分泌IFN-γ、IL-2、TNF-α等多种细胞因子的T细胞的水平上升,促进了特异性细胞免疫应答,从而有效杀伤靶细胞[40]。
4.3 自噬促进剂一项加入自噬促进剂Tat-beclin 1的全肿瘤细胞疫苗dMNs的研究表明,上调细胞自噬水平可将未成熟的树突状细胞募集到皮肤的给药部位,促进其成熟并迁移到淋巴结,进而将抗原肽呈递给T细胞以触发抗肿瘤免疫应答[41]。
5 dMNs疫苗临床试验进展目前,针对dMNs疫苗的研究大多仍停留在动物实验阶段,只开展了少部分主要针对安全性[42]和大众接受度[43-44]的临床试验,并未有疫苗上市。Rouphael等[44]针对流感dMNs疫苗的免疫效果进行了随机I期临床试验,结果表明dMNs组在接种后第28天的血清转化率显著高于安慰剂组,且dMNs诱导的体液和细胞免疫反应与肌肉注射疫苗相似或更强[45]。这一发现与动物实验的结果一致,说明使用dMNs进行皮肤疫苗接种可以引起机体强烈的免疫应答[46-47],且达到与肌肉注射相当的免疫效果。而另一项关于麻疹风疹疫苗的dMNs研究(临床编号202008836432905)正在进行当中[48],其实验数据尚未公布但值得期待。
6 难点与展望dMNs作为一种疫苗递送工具,具有诸多优点,包括能够减轻接种过程中的疼痛感,节省抗原剂量,避免交叉感染,减轻医务人员的操作负担,并且大众接受度高[43],有利于疫苗的普及。但无可否认,dMNs的实际应用仍存在一些问题尚未解决:①dMNs疫苗引起的免疫应答受到疫苗种类、浓度以及添加佐剂种类等因素的影响,因此,有必要进一步探究不同种类的疫苗是否均能在人体引起与肌肉注射相当甚至更强的免疫反应;②如何在使用更少抗原的情况下引起更强的体液和细胞免疫应答;③是否添加佐剂、添加佐剂的种类以及添加浓度以增强dMNs疫苗的免疫效果;④针尖溶解的时间、皮肤的过敏反应、储存过程中疫苗的稳定性以及每次可递送入皮内的剂量。以上都是制作dMNs疫苗时需要考虑的问题。
总而言之,目前dMNs疫苗的研究已经取得了一定进展,有广泛的应用前景。相信在未来,通过进一步的完善,dMNs疫苗有望发展为一种更安全、便捷、高效、经济的产品,从而提高发展中国家疫苗的接种率,为除人类之病痛贡献一份力量。
| [1] |
Babiuk S, Baca-Estrada M, Babiuk LA, Ewen C, Foldvari M. Cutaneous vaccination: the skin as an immunologically active tissue and the challenge of antigen delivery[J]. J Control Release, 2000, 66(2/3): 199-214.
|
| [2] |
Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system[J]. J Pharm Pharmacol, 2012, 64(1): 11-29.
|
| [3] |
Jin X, Zhu DD, Chen BZ, Ashfaq M, Guo XD. Insulin delivery systems combined with microneedle technology[J]. Adv Drug Deliv Rev, 2018, 127: 119-137.
[DOI]
|
| [4] |
Shende P, Sardesai M, Gaud RS. Micro to nanoneedles: a trend of modernized transepidermal drug delivery system[J]. Artif Cells Nanomed Biotechnol, 2018, 46(1): 19-25.
[DOI]
|
| [5] |
Lee D, Li CG, Ihm C, Jung H. A three-dimensional and bevel-angled ultrahigh aspect ratio microneedle for minimally invasive and painless blood sampling[J]. Sensors and Actuators B: Chemical, 2018, 255: 384-390.
[DOI]
|
| [6] |
Hwa KY, Chang V, Cheng YY, Wang YD, Jan PS, Subramani B, Wu MJ, Wang BK. Analyzing polymeric matrix for fabrication of a biodegradable microneedle array to enhance transdermal delivery[J]. Biomed Microdevices, 2017, 19(4): 84.
[DOI]
|
| [7] |
Sullivan SP, Koutsonanos DG, Del PMM, Lee JW, Zarnitsyn V, Choi SO, Murthy N, Compans RW, Skountzou I, Prausnitz MR. Dissolving polymer microneedle patches for influenza vaccination[J]. Nat Med, 2010, 16(8): 915-920.
[DOI]
|
| [8] |
沈瑞雪, 朱壮志, 章俊云, 罗华菲. 可溶性微针在经皮给药系统中的开发进展[J]. 世界临床药物, 2017, 38(9): 638-642. |
| [9] |
Moon SS, Richter-Roche M, Resch TK, Wang Y, Foytich KR, Wang H, Mainou BA, Pewin W, Lee J, Henry S, McAllister DV, Jiang B. Microneedle patch as a new platform to effectively deliver inactivated polio vaccine and inactivated rotavirus vaccine[J]. NPJ Vaccines, 2022, 7(1): 26.
[DOI]
|
| [10] |
Zhao B, Jin Z, Yu Y, Li Y, Wang J, Wan W, Hu C, Li X, Li Y, Xin W, Kang L, Yang H, Wang J, Gao S. A thermostable dissolving microneedle vaccine with recombinant protein of botulinum neurotoxin serotype A[J]. Toxins (Basel), 2022, 14(12): 881.
[DOI]
|
| [11] |
Tian Y, Lee J, van der Maaden K, Bhide Y, de Vries-Idema JJ, Akkerman R, O'Mahony C, Jiskoot W, Frijlink HW, Huckriede A, Hinrichs W, Bouwstra JA, Beukema M. Intradermal administration of influenza vaccine with trehalose and pullulan-based dissolving microneedle arrays[J]. J Pharm Sci, 2022, 111(4): 1070-1080.
[DOI]
|
| [12] |
Schepens B, Vos PJ, Saelens X, van der Maaden K. Vaccination with influenza hemagglutinin-loaded ceramic nanoporous microneedle arrays induces protective immune responses[J]. Eur J Pharm Biopharm, 2019, 136: 259-266.
[DOI]
|
| [13] |
Raphael AP, Prow TW, Crichton ML, Chen X, Fernando GJ, Kendall MA. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays[J]. Small, 2010, 6(16): 1785-1793.
[DOI]
|
| [14] |
Moon S, Wang Y, Edens C, Gentsch JR, Prausnitz MR, Jiang B. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch[J]. Vaccine, 2013, 31(34): 3396-3402.
[DOI]
|
| [15] |
Li Y, Hu X, Dong Z, Chen Y, Zhao W, Wang Y, Zhang L, Chen M, Wu C, Wang Q. Dissolving microneedle arrays with optimized needle geometry for transcutaneous immunization[J]. Eur J Pharm Sci, 2020, 151: 105361.
[DOI]
|
| [16] |
Lee MH, Seo H, Lee MS, Kim BJ, Kim HL, Lee DH, Oh J, Shin JY, Jin JY, Jeong DH, Kim BJ. Protection against tuberculosis achieved by dissolving microneedle patches loaded with live Mycobacterium paragordonae in a bcg prime-boost strategy[J]. Front Immunol, 2023, 14: 1178688.
[DOI]
|
| [17] |
Beaver JT, Mills LK, Swieboda D, Lelutiu N, Esser ES, Antao OQ, Scountzou E, Williams DT, Papaioannou N, Littauer EQ, Romanyuk A, Compans RW, Prausnitz MR, Skountzou I. Cutaneous vaccination ameliorates Zika virus-induced neuro-ocular pathology via reduction of anti-ganglioside antibodies[J]. Hum Vaccin Immunother, 2020, 16(9): 2072-2091.
[DOI]
|
| [18] |
Yan Q, Cheng Z, Liu H, Shan W, Cheng Z, Dai X, Xue Y, Chen F. Enhancement of Ag85B DNA vaccine immunogenicity against tuberculosis by dissolving microneedles in mice[J]. Vaccine, 2018, 36(30): 4471-4476.
[DOI]
|
| [19] |
Fan F, Zhang X, Zhang Z, Ding Y, Wang L, Xu X, Pan Y, Gong FY, Jiang L, Kang L, Ha Z, Lu H, Hou J, Kou Z, Zhao G, Wang B, Gao XM. Potent immunogenicity and broad-spectrum protection potential of microneedle array patch-based Covid-19 DNA vaccine candidates encoding dimeric RBD chimera of SARS-Cov and SARS-Cov-2 variants[J]. Emerg Microbes Infect, 2023, 12(1): 2202269.
[DOI]
|
| [20] |
Ding Y, Fan F, Xu X, Zhao G, Zhang X, Zhao H, Wang L, Wang B, Gao XM. A Covid-19 DNA vaccine candidate elicits broadly neutralizing antibodies against multiple SARS-Cov-2 variants including the currently circulating omicron Ba.5, Bf.7, Bq.1 and Xbb[J]. Vaccines (Basel), 2023, 11(4): 778.
[DOI]
|
| [21] |
Zhu Z, Ye X, Ku Z, Liu Q, Shen C, Luo H, Luan H, Zhang C, Tian S, Lim C, Huang Z, Wang H. Transcutaneous immunization via rapidly dissolvable microneedles protects against hand-foot-and-mouth disease caused by enterovirus 71[J]. J Control Release, 2016, 243: 291-302.
[DOI]
|
| [22] |
Lee SJ, Lee HS, Hwang YH, Kim JJ, Kang KY, Kim SJ, Kim HK, Kim JD, Jeong DH, Paik MJ, Yee ST. Enhanced anti-tumor immunotherapy by dissolving microneedle patch loaded ovalbumin[J]. PLoS One, 2019, 14(8): e220382.
[DOI]
|
| [23] |
DeMuth PC, Min Y, Irvine DJ, Hammond PT. Implantable silk composite microneedles for programmable vaccine release kinetics and enhanced immunogenicity in transcutaneous immunization[J]. Adv Healthc Mater, 2014, 3(1): 47-58.
[DOI]
|
| [24] |
Liu S, Zhang S, Duan Y, Niu Y, Gu H, Zhao Z, Zhang S, Yang Y, Wang X, Gao Y, Yang P. Transcutaneous immunization of recombinant staphylococcal enterotoxin B protein using a dissolving microneedle provides potent protection against lethal enterotoxin challenge[J]. Vaccine, 2019, 37(29): 3810-3819.
[DOI]
|
| [25] |
Guo L, Chen J, Qiu Y, Zhang S, Xu B, Gao Y. Enhanced transcutaneous immunization via dissolving microneedle array loaded with liposome encapsulated antigen and adjuvant[J]. Int J Pharm, 2013, 447(1/2): 22-30.
|
| [26] |
Vassilieva EV, Li S, Korniychuk H, Taylor DM, Wang S, Prausnitz MR, Compans RW. Cgamp/Saponin adjuvant combination improves protective pesponse to influenza vaccination by microneedle patch in an aged mouse model[J]. Front Immunol, 2020, 11: 583251.
[DOI]
|
| [27] |
Leone M, Romeijn S, Du G, Le Devedec SE, Vrieling H, O'Mahony C, Bouwstra JA, Kersten G. Diphtheria toxoid dissolving microneedle vaccination: adjuvant screening and effect of repeated-fractional dose administration[J]. Int J Pharm, 2020, 580: 119182.
[DOI]
|
| [28] |
Takeda K, Akira S. Toll-like receptors in innate immunity[J]. Int Immunol, 2005, 17(1): 1-14.
|
| [29] |
Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E. Unleashing the potential of NOD-and toll-like agonists as vaccine adjuvants[J]. Proc Natl Acad Sci USA, 2014, 111(34): 12294-12299.
[DOI]
|
| [30] |
Vreman S, Rebel J, McCaffrey J, Ledl K, Arkhipova K, Collins D, McDaid D, Savelkoul H, Skovgaard K, Moore AC, Stockhofe-Zurwieden N. Early immune responses in skin and lymph node after skin delivery of toll-like receptor agonists in neonatal and adult pigs[J]. Vaccine, 2021, 39(13): 1857-1869.
[DOI]
|
| [31] |
Kaur A, Baldwin J, Brar D, Salunke DB, Petrovsky N. Toll-like receptor (Tlr) agonists as a driving force behind next-generation vaccine adjuvants and cancer therapeutics[J]. Curr Opin Chem Biol, 2022, 70: 102172.
[DOI]
|
| [32] |
Zheng X, Zhu J, Zheng C, Tan Z, Ji Z, Tao J, Zhao Y, Ji Z, Hu Y. Dissolving microneedle arrays as a hepatitis B vaccine delivery system adjuvanted by APC-targeted poly (Lactic-Co-Glycolic Acid) (PLGA) nanoparticles[J]. AAPS PharmSciTech, 2023, 24(1): 42.
[DOI]
|
| [33] |
吴雅琦, 李蒙, 邢昊楠, 陈大全, 郑爱萍. 基于PLGA纳米粒的SARS-Cov-2 DNA疫苗的制备及初步评价[J]. 药学学报, 2023, 58(6): 1677-1684. |
| [34] |
黄佳璐. 新型冠状病毒rS1-E-PLGA多价纳米疫苗的研发与免疫效果评价[D]. 衡阳: 南华大学, 2022.
|
| [35] |
Koerner J, Horvath D, Herrmann VL, MacKerracher A, Gander B, Yagita H, Rohayem J, Groettrup M. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy[J]. Nat Commun, 2021, 12(1): 2935.
[DOI]
|
| [36] |
万伟. 一种基于PLGA纳米颗粒的产气荚膜梭菌E毒素可溶性微针疫苗[D]. 北京: 北京化工大学, 2023.
|
| [37] |
Duong H, Yin Y, Thambi T, Kim BS, Jeong JH, Lee DS. Highly potent intradermal vaccination by an array of dissolving microneedle polypeptide cocktails for cancer immunotherapy[J]. J Mater Chem B, 2020, 8(6): 1171-1181.
[DOI]
|
| [38] |
Balmert SC, Carey CD, Falo GD, Sethi SK, Erdos G, Korkmaz E, Falo LJ. Dissolving undercut microneedle arrays for multicomponent cutaneous vaccination[J]. J Control Release, 2020, 317: 336-346.
[DOI]
|
| [39] |
Li Z, He Y, Deng L, Zhang ZR, Lin Y. A fast-dissolving microneedle array loaded with chitosan nanoparticles to evoke systemic immune responses in mice[J]. J Mater Chem B, 2020, 8(2): 216-225.
[DOI]
|
| [40] |
Li M, Yang L, Wang C, Cui M, Wen Z, Liao Z, Han Z, Zhao Y, Lang B, Chen H, Qian J, Shu Y, Zeng X, Sun C. Rapid induction of long-lasting systemic and mucosal immunity via thermostable microneedle-mediated chitosan oligosaccharide-encapsulated DNA nanoparticles[J]. ACS Nano, 2023, 17(23): 24200-24217.
[DOI]
|
| [41] |
杨丹. 工程化全细胞疫苗可溶微针贴片联合自噬调控策略增强肿瘤免疫治疗研究[D]. 广州: 暨南大学, 2022.
|
| [42] |
Hirobe S, Azukizawa H, Matsuo K, Zhai Y, Quan YS, Kamiyama F, Suzuki H, Katayama I, Okada N, Nakagawa S. Development and clinical study of a self-dissolving microneedle patch for transcutaneous immunization device[J]. Pharm Res, 2013, 30(10): 2664-2674.
[DOI]
|
| [43] |
Frew PM, Paine MB, Rouphael N, Schamel J, Chung Y, Mulligan MJ, Prausnitz MR. Acceptability of an inactivated influenza vaccine delivered by microneedle patch: results from a phase I clinical trial of safety, reactogenicity, and immunogenicity[J]. Vaccine, 2020, 38(45): 7175-7181.
[DOI]
|
| [44] |
Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, Pewin W, Frew PM, Yu T, Thornburg NJ, Kabbani S, Lai L, Vassilieva EV, Skountzou I, Compans RW, Mulligan MJ, Prausnitz MR. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase I trial[J]. Lancet, 2017, 390(10095): 649-658.
[DOI]
|
| [45] |
Rouphael NG, Lai L, Tandon S, McCullough MP, Kong Y, Kabbani S, Natrajan MS, Xu Y, Zhu Y, Wang D, O'Shea J, Sherman A, Yu T, Henry S, McAllister D, Stadlbauer D, Khurana S, Golding H, Krammer F, Mulligan MJ, Prausnitz MR. Immunologic mechanisms of seasonal influenza vaccination administered by microneedle patch from a randomized phase I trial[J]. NPJ Vaccines, 2021, 6(1): 89.
[DOI]
|
| [46] |
Skountzou I, Compans RW. Skin immunization with influenza vaccines[J]. Curr Top Microbiol Immunol, 2015, 386: 343-369.
|
| [47] |
Marshall S, Sahm LJ, Moore AC. The success of microneedle-mediated vaccine delivery into skin[J]. Hum Vaccin Immunother, 2016, 12(11): 2975-2983.
[DOI]
|
| [48] |
Adigweme I, Akpalu E, Yisa M, Donkor S, Jarju LB, Danso B, Mendy A, Jeffries D, Njie A, Bruce A, Royals M, Goodson J L, Prausnitz MR, McAllister D, Rota PA, Henry S, Clarke E. Study protocol for a phase 1/2, single-centre, double-blind, double-dummy, randomized, active-controlled, age de-escalation trial to assess the safety, tolerability and immunogenicity of a measles and rubella vaccine delivered by a microneedle patch in healthy adults (18 to 40 years), measles and rubella vaccine-primed toddlers (15 to 18 months) and measles and rubella vaccine-naive infants (9 to 10 months) in the gambia [Measles and rubella vaccine microneedle patch phase 1/2 age de-escalation trial][J]. Trials, 2022, 23(1): 775.
[DOI]
|
 2024, Vol. 19
2024, Vol. 19


