2. 复旦大学附属华山医院感染科,上海 200040
2. Department of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai 200040, China
结核病是由结核分枝杆菌(Mycobacterium tuberculosis)引起的主要经呼吸道传播的传染性疾病,全世界约1/3的人口感染过结核分枝杆菌,每年有1 000多万新发病例和180万死亡病例[1]。大多数结核分枝杆菌感染不会发展成为活动性结核病,而是呈现潜伏结核感染(latent tuberculosis infection,LTBI)状态,仅5%~10%会发展成为活动性结核病[2],提示结核病的发生与宿主的免疫状态有关,宿主在机体感染结核分枝杆菌时提供的免疫保护作用不足或宿主基因对结核分枝杆菌的高易感性可能是结核病发生发展的关键。目前,诸多研究认为人类基因多态性与结核易感性有关,如人类白细胞抗原(human leucocyte antigen,HLA)[3]、某些细胞因子及其受体[4-6]的基因多态性可影响人群的结核易感性。白细胞介素22(interleukin 22,IL-22)是IL-10家族成员之一,主要由T细胞和自然杀伤(natural killer, NK)细胞产生[7-8]。在宿主对抗结核分枝杆菌感染过程中,IL-22能通过巨噬细胞等途径为机体提供重要免疫保护作用[9-11]。因此,推测人类IL-22基因的多态性与人群结核易感性有较大相关性。本研究对结核病组、LTBI组、健康对照组人群的IL-22基因单核苷酸多态性(single nucleotide polymorphism,SNP)进行分析,以探究IL-22基因SNP与人群结核易感性的关系。
1 材料与方法 1.1 研究对象选取2006年11月—2008年9月重庆市肺科医院收治并确诊的肺结核患者453例为结核病组,其中女133例,男320例,年龄11~88岁,平均(48±20)岁。结核病按国家统一标准确诊:即痰菌阳性同时胸部X线片有肺结核特征病灶;或痰菌阴性,但胸部X线片示活动性肺结核征象、结核菌素纯蛋白衍化物(purified protein derivative,PPD)试验强阳性并具有明显结核病临床体征。所有病例均排除肺炎、肺癌、尘肺等具有结核相似病症者,同时排除人类免疫缺陷病毒(human immunodeficiency virus,HIV)感染、糖尿病、肿瘤、长期使用激素、器官移植等免疫功能低下者。健康对照组和LTBI组为结核病组患者的长期密切接触者,共373名。对照组共264例,其中女143例,男121例,年龄3~76岁,平均(37±14)岁。对照组经胸透排除肺结核病史,经PPD试验排除其他部位结核,T-SPOT.TB检测阴性,同时排除具有慢性病病史及接受激素治疗所致免疫功能低下者。LTBI组共109例,其中女66例,男43例,年龄3~78岁,平均(42±15)岁。LTBI组无临床症状,胸部X线片排除活动性肺结核,T-SPOT.TB检测阳性,同时排除具有慢性病病史及接受激素治疗所致免疫功能低下者。研究对象均为汉族,均签署了知情同意书。
1.2 主要仪器和试剂主要仪器包括PCR Thermal Cycler Dice仪(日本TaKaRa公司)、台式离心机(德国Eppendorf公司)、CO2细胞培养箱(上海一恒科技有限公司)、ABI 3730XL测序仪。主要试剂有QIAamp® DNA Blood Mini Kit(美国QIAGEN公司)、T-SPOT. TB试剂盒(上海复星长征医学科学有限公司)、淋巴细胞分离液(Amersham,Piscataway公司)、红细胞裂解液(eBioscience公司)、SNPscanTM多重SNP分型试剂盒。
1.3 标本采集和DNA提取采用美国BD公司抗凝真空采血管采集外周静脉血8 mL。采用全血DNA抽提试剂盒提取DNA,严格按说明书进行操作,抽提的DNA置于-80 ℃冰箱储存备用。
1.4 T-SPOT.TB检测采用T-SPOT.TB试剂盒,根据产品说明书进行操作。在包被小鼠抗人γ干扰素(interferon γ,INF-γ)单克隆抗体的96孔培养板中,每孔孵育2.5×105个人外周血单核细胞(peripheral blood mononuclear cell,PBMC),分别加入AIM-V培养基(阴性对照)、早期分泌抗原靶6 kDa蛋白(early secreted antigenic target of 6 kDa,ESAT-6;A孔)、培养滤液蛋白10(culture filtrate protein of 10 kDa,CFP-10;B孔)和植物血凝素(phytohaemagglutinin,PHA;阳性对照),孵育16~24 h后显色。用酶联斑点免疫自动读板器(德国AID-GmbH)根据预先设定的点的大小和强度,自动计数每孔点数。如果A孔和(或)B孔的点数减去阴性孔点数≥6个,且≥2倍阴性孔点数,则T-SPOT.TB结果判为阳性,否则判为阴性。所有结果均复查,必要时人工校正计数。
1.5 SNP测定和分型从HapMap数据库选取IL-22基因的4个标签SNP,分别为rs1179249、rs2227491、rs17224704、rs2227478。采用SNPscanTM多重SNP分型技术,步骤如下。样本裂解:取4 μL DNA样本(DNA浓度30~50 ng/μL),分别加入2.5 μL 4×DNA Lysis Buffer,用水补足至10 μL,盖好膜,振荡混匀,离心,于PCR仪98 ℃变性5 min,立即冰置。连接反应:在冰置的降解DNA样本中,加入10 μL连接反应预混合液,盖上盖膜,轻微振荡混匀,3 000 r/min离心30 s,然后立即将离心后的96孔板置于PCR仪,去除盖膜,盖上96孔橡胶盖垫,盖好PCR仪热盖。按以下程序运行:94 ℃ 1 min,58 ℃ 4 h,共4个循环;94 ℃ 1 min,最终72 ℃延伸。多重荧光PCR:取新96孔板放置于冰上,每孔分装19 μL PCR预混合液,将上述连接产物每孔取1 μL加入该96孔板对应位置,盖上盖膜,轻微振荡混匀,3 000 r/min离心30 s,然后立即将离心后的96孔板置于PCR仪,去除盖膜,盖上96孔橡胶盖垫,盖好PCR仪热盖。按以下程序运行:95 ℃ 2 min,94 ℃ 20 s,62 ℃ 40 s,72 ℃ 1.5 min,-0.5 ℃/循环,共9个循环;然后94 ℃ 20 s,57 ℃ 40 s,72 ℃ 1.5 min,共25个循环;68 ℃ 1 h,最终4 ℃延伸。PCR产物稀释10倍后,取1 μL与0.5 μL Liz500 Size Standard、8.5 μL Hi-Di混匀,95 ℃变性5 min后,采用ABI 3730XL测序仪测序。
1.6 统计学分析使用SPSS软件对4个SNP位点进行Logistic回归分析,包括按基因型分类的共显性模型和按等位基因分类,并按年龄(≤25岁、26~55岁、>55岁)分层重复以上分析。
2 结果 2.1 T-SPOT.TB检测结果采用T-SPOT. TB试剂盒对373例肺结核患者的密切接触者进行检测。从全血分离淋巴细胞,用ESAT-6与CFP-10两种抗原刺激。结果表明,109例呈现T-SPOT.TB阳性,入LTBI组;264例呈现T-SPOT.TB阴性,入健康对照组。
2.2 哈温平衡(Hardy-Weinberg equilibrium,HWE)检验使用Haploview软件对研究对象进行HWE检测,各基因型频率分布符合HWE遗传平衡定律,P>0.05,具有群体代表性。
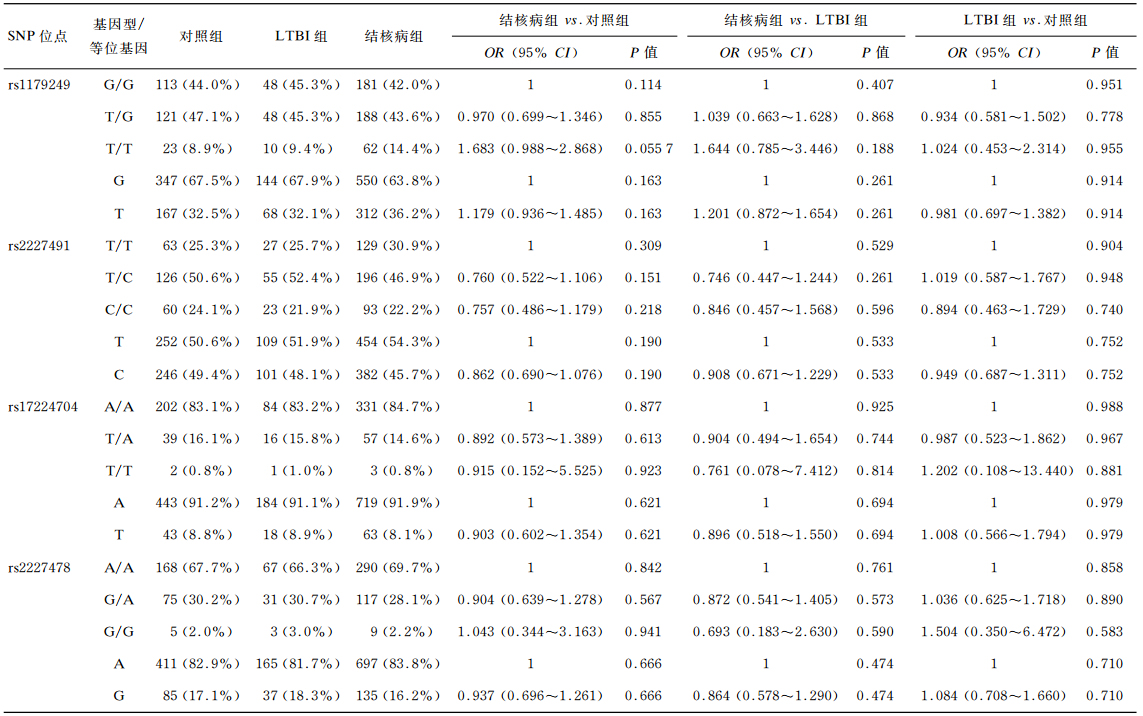
2.3 IL-22基因型与肺结核易感性的关联分析IL-22基因4个SNP位点rs1179249、rs2227491、rs17224704、rs2227478的基因型和等位基因频率结果见表 1。结果显示,4个SNP位点的基因型和等位基因分布在3组的组内及组间比较差异均无统计学意义(P>0.05)。进一步按年龄分层分析发现(表 2),>55岁人群中,rs17224704位点TA基因型在对照组中的分布显著高于结核病组(P=0.047 9,OR=0.365,95% CI=0.135~0.991);AA基因型在LTBI组中的分布显著低于结核病组(P=0.027 6);TA基因型在LTBI组中的分布显著高于结核病组(P=0.007 37,OR=0.213,95% CI=0.069~0.660)。对照组和LTBI组的等位基因T频率显著高于结核病组(P=0.026 9,OR=0.388,95% CI=0.167~0.897;P=0.025 0,OR=0.322,95% CI=0.119~0.867)。
|

|
据世界卫生组织(World Health Organization,WHO)的资料,全球约32%的人口感染结核分枝杆菌,仅1/10的感染者会进一步发展为结核病[12],提示个体差异可能与结核病遗传易感性有关。国内外也不断报道了一些基因(如NRAMP1、MBL、TLR、HLA、VDR、IFN-γ、IL-1R、IL-6、FOXP3、SP110等)与结核病宿主遗传易感性的研究结果。Wilkinson等[13]发现,VDR基因Fok I多态体的ff基因型及25-羟维生素D3缺乏与居住在伦敦的古吉拉特印度人肺结核有很强的关联性。在巴西人群中,IL17A-197和IL6-174位点的基因多态性与结核易感性也存在关联[14]。IL-22是2000年Renauld小组用IL-9刺激一株T淋巴瘤细胞系时首次发现的,其在结构上与IL-10有23%的同源性,被列入IL-10家族成员[15]。IL-22在感染和自身免疫性疾病中扮演着重要角色,可促进抗微生物肽生成,抵抗细菌感染[16]。IL-22被抗体中和后的小鼠更易患铜绿假单胞菌肺炎,且在感染铜绿假单胞菌后引起更严重的肺部损伤[17]。在非人灵长类动物模型中,结核分枝杆菌感染可导致合成IL-22的T细胞数量明显升高,表明表达IL-22的T细胞参与了灵长类结核病的免疫调控过程[18-19]。还有研究表明,在体外结核分枝杆菌感染巨噬细胞模型中,IL-15和IL-23等细胞因子可刺激NK细胞分泌大量IL-22,其能促进吞噬溶酶体的融合,显著增强巨噬细胞对结核分枝杆菌的胞内清除作用[11]。鉴于IL-22的重要生物学作用,其基因多态性与疾病的遗传易感性成为研究热点。文献报道,IL-22基因的rs2046068、rs2227478、rs2227485、rs11611206和rs1179251位点多态性与自身免疫性甲状腺疾病相关[20]。IL-22基因拷贝数变异与强直性脊柱炎有关,较低的拷贝数是强直性脊柱炎的保护性因素之一[21]。rs1179251位点的多态性与狼疮肾炎相关[22]。rs2227472位点的多态性与肝癌易感性也相关,其多态性能影响PBMC的IL-22分泌,从而影响肝癌的免疫学机制[23]。另有研究表明,IL-22基因启动子区域的rs2227473位点与结核易感性相关,此位点的等位基因A使机体IL-22表达水平更高[24]。
本研究显示,IL-22基因的4个SNP位点rs1179249、rs2227491、rs17224704、rs2227478的基因型分布在3组的组内及组间比较均未发现显著性差异(P>0.05)。进一步按年龄分层分析发现,>55岁人群中,rs17224704位点TA基因型在对照组中的分布显著高于结核病组(P=0.047 9,OR=0.365,95% CI=0.135~0.991);AA基因型在LTBI组中的分布显著低于结核病组(P=0.027 6);TA基因型在LTBI组中的分布显著高于结核病组(P=0.007 37,OR=0.213,95% CI=0.069~0.660)。对照组和LTBI组的等位基因T频率显著高于结核病组(P=0.026 9,OR=0.388,95% CI=0.167~0.897;P=0.025 0,OR=0.322,95% CI=0.119~0.867)。结果表明,在中国重庆地区的汉族人群中,IL-22基因rs17224704位点的多态性与肺结核易感性可能显著相关,且呈年龄依赖性,等位基因T可能是肺结核的保护性基因。
已有研究表明,IL-22可通过多种途径参与抗结核免疫。产生IL-22的细胞主要来源于CD4+ T细胞、CD8+ T细胞、γδ T细胞等[25-26]。IL-22通过上调Rab7表达和下调Rab14诱导钙粒蛋白A(calgranulin A)表达,抑制结核分枝杆菌的胞内生长[27]。也有研究表明,IL-22基因启动子区rs2227473的多态性影响IL-22表达,对机体抗结核免疫造成影响[28]。Zhang等研究发现,成功的抗结核治疗能通过降低CD19+ CD5+ CD1d+调节性B细胞的频率来诱导增强持续的结核抗原特异性的IL-22免疫应答[29]。虽然IL-22参与抗结核免疫,但其到底起免疫保护性作用还是免疫病理性作用,目前研究结论尚不一致。这可能与结核分枝杆菌感染和宿主发病不同阶段有关,也可能与同一宿主不同组织中IL-22的应答水平不一致有关[18, 30],需进一步探索。结核病的发生是结核分枝杆菌和宿主基因调控免疫应答共同作用的结果,也是一个非常复杂的过程。本研究揭示SNP位点rs17224704的多态性与肺结核易感性存在相关性,但该位点的多态性如何影响IL-22的表达水平进而影响机体免疫功能,基因调控途径和机制如何,尤其是在结核分枝杆菌感染不同状态下,分泌IL-22的T细胞各亚群的免疫学功能特征又是什么,均有待进一步研究。
| [1] |
WHO. Tuberculosis (TB)[EB/OL]. http://www.who.int/tb/en.
|
| [2] |
Ahmad S. Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection[J]. Clin Dev Immunol, 2011.
[DOI]
|
| [3] |
Yim JJ, Selvaraj P. Genetic susceptibility in tuberculosis[J]. Respirology, 2010, 15(2): 241-256.
[DOI]
|
| [4] |
Lio D, Marino V, Serauto A, Gioia V, Scola L, Crivello A, Forte GI, Colonna-Romano G, Candore G, Caruso C. Genotype frequencies of the +874T->A single nucleotide polymorphism in the first intron of the interferon-gamma gene in a sample of Sicilian patients affected by tuberculosis[J]. Eur J Immunogenet, 2002, 29(5): 371-374.
[DOI]
|
| [5] |
Rossouw M, Nel HJ, Cooke GS, van Helden PD, Hoal EG. Association between tuberculosis and a polymorphic NFkappaB binding site in the interferon gamma gene[J]. Lancet, 2003, 361(9372): 1871-1872.
[DOI]
|
| [6] |
Tso HW, Lau YL, Tam CM, Wong HS, Chiang AK. Associations between IL12B polymorphisms and tuberculosis in the Hong Kong Chinese population[J]. J Infect Dis, 2004, 190(5): 913-919.
[DOI]
|
| [7] |
Luci C, Reynders A, Ivanov Ⅱ, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, Dalod M, Littman DR, Vivier E, Tomasello E. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin[J]. Nat Immunol, 2009, 10(1): 75-82.
[DOI]
|
| [8] |
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease[J]. Immunity, 2008, 29(6): 947-957.
[DOI]
|
| [9] |
Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, Hanekom WA. Distinct, specific IL-17-and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response[J]. J Immunol, 2008, 180(3): 1962-1970.
[DOI]
|
| [10] |
Zeng G, Chen CY, Huang D, Yao S, Wang RC, Chen ZW. Membrane-bound IL-22 after de novo production in tuberculosis and anti-Mycobacterium tuberculosis effector function of IL-22+ CD4+ T cells[J]. J Immunol, 2011, 187(1): 190-199.
[DOI]
|
| [11] |
Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, Vankayalapati R. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion[J]. J Immunol, 2009, 183(10): 6639-6645.
[DOI]
|
| [12] |
Tso HW, Ip WK, Chong WP, Tam CM, Chiang AK, Lau YL. Association of interferon gamma and interleukin 10 genes with tuberculosis in Hong Kong Chinese[J]. Genes Immun, 2005, 6(4): 358-363.
[DOI]
|
| [13] |
Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study[J]. Lancet, 2000, 355(9204): 618-621.
[DOI]
|
| [14] |
Milano M, Moraes MO, Rodenbusch R, Carvalho CX, Delcroix M, Mousquer G, Laux da Costa L, Unis G, Dalla Costa ER, Rossetti ML. Single nucleotide polymorphisms in IL17A and IL6 are associated with decreased risk for pulmonary tuberculosis in Southern Brazilian population[J]. PLoS One, 2016, 11(2): e147814.
[PMC]
|
| [15] |
Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9[J]. J Immunol, 2000, 164(4): 1814-1819.
[DOI]
|
| [16] |
Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia[J]. Nat Med, 2008, 14(3): 275-281.
[DOI]
|
| [17] |
Broquet A, Jacqueline C, Davieau M, Besbes A, Roquilly A, Martin J, Caillon J, Dumoutier L, Renauld JC, Heslan M, Josien R, Asehnoune K. Interleukin-22 level is negatively correlated with neutrophil recruitment in the lungs in a Pseudomonas aeruginosa pneumonia model[J]. Sci Rep, 2017, 7(1): 11010.
[DOI]
|
| [18] |
Yao S, Huang D, Chen CY, Halliday L, Zeng G, Wang RC, Chen ZW. Differentiation, distribution and gammadelta T cell-driven regulation of IL-22-producing T cells in tuberculosis[J]. PLoS Pathog, 2010, 6(2): e1000789.
[DOI]
|
| [19] |
Huang D, Chen CY, Zhang M, Qiu L, Shen Y, Du G, Zhou K, Wang R, Chen ZW. Clonal immune responses of Mycobacterium-specific γδ T cells in tuberculous and non-tuberculous tissues during M. tuberculosis infection[J]. PLoS One, 2012, 7(2): e30631.
[DOI]
|
| [20] |
Song RH, Li Q, Wang W, Yao QM, Shao XQ, Zhang JA. Variants of interleukin-22 gene confer predisposition to autoimmune thyroid disease[J]. Int J Endocrinol, 2017.
[DOI]
|
| [21] |
Zhang X, Li X, Han R, Chen M, Yuan Y, Hu X, Wang M, Li R, Yang X, Xia Q, Ma Y, Yang J, Tong J, Xu S, Xu J, Shuai Z, Pan F. Copy number variations of the IL-22 gene are associated with ankylosing spondylitis: A case-control study in Chinese Han population[J]. Hum Immunol, 2017, 78(9): 547-552.
[DOI]
|
| [22] |
祝艳. IL-22及其受体与系统性红斑狼疮的关联性研究[D]. 安徽医科大学, 2014.
|
| [23] |
何晓, 王小华, 邱振纲, 陈心春. IL-22基因单核苷酸多态性与肝癌易感性的相关性研究[J]. 赣南医学院学报, 2013, 33(1): 29-32. [CNKI]
|
| [24] |
Zhang G, Chen X, Chan L, Zhang M, Zhu B, Wang L, Zhu X, Zhang J, Zhou B, Wang J. An SNP selection strategy identified IL-22 associating with susceptibility to tuberculosis in Chinese[J]. Sci Rep, 2011, 1: 20.
[DOI]
|
| [25] |
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis[J]. Nature, 2007, 445(7128): 648-651.
[DOI]
|
| [26] |
Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens[J]. Nat Med, 2008, 14(3): 282-289.
[DOI]
|
| [27] |
Dhiman R, Venkatasubramanian S, Paidipally P, Barnes PF, Tvinnereim A, Vankayalapati R. Interleukin 22 inhibits intracellular growth of Mycobacterium tuberculosis by enhancing calgranulin A expression[J]. J Infect Dis, 2014, 209(4): 578-587.
[DOI]
|
| [28] |
Ding GG, Zhang GL, Chen XC, Zhang MX, Yang L, Wang Z. A study on the relationship between single nucleotide polymorphisms of interleukin-22 and susceptibility to pulmonary tuberculosis[J]. Zhonghua Jie He He Hu Xi Za Zhi, 2012, 35(8): 596-600.
[URI]
|
| [29] |
Zhang M, Zeng G, Yang Q, Zhang J, Zhu X, Chen Q, Suthakaran P, Zhang Y, Deng Q, Liu H, Zhou B, Chen X. Anti-tuberculosis treatment enhances the production of IL-22 through reducing the frequencies of regulatory B cell[J]. Tuberculosis (Edinb), 2014, 94(3): 238-244.
[DOI]
|
| [30] |
Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection[J]. Cytokine Growth Factor Rev, 2010, 21(5): 365-379.
[DOI]
|
 2018, Vol. 13
2018, Vol. 13


