2. 第二军医大学微生物学教研室,上海 200433
2. Department of Microbiology, The Second Military Medical University, Shanghai 200433, China
C型凝集素受体(C-type lectin receptor,CLR)调节病毒感染与病毒种类和易感细胞存在的其他病毒特异性受体密切相关。来源不同的CLR如树突细胞特异性细胞间黏附分子-3结合非整合素分子(dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin,DC-SIGN)、肝/淋巴结特异性细胞间黏附分子-3结合非整合素分子(liver/lymph node-specific intercellular adhesion molecules-3-grabbing non-integrin, L-SIGN)和LSECtin(liver and lymph node sinusoidal endothelial cell C-type lectin)均能与病毒表面糖蛋白相互作用,协助多种病毒感染和扩散,包括疱疹病毒、反转录病毒、正黏和副黏病毒、冠状病毒及大多数出血热病毒和脑炎病毒等[1]。病毒对CLR的依赖程度也不同。如DC-SIGN是决定登革热病毒感染DC并在其中复制的受体[2],但对丙型肝炎病毒(hepatitis C virus,HCV)而言只是携带和转运的辅助性受体[3]。对大多数病原体而言,DC-SIGN主要表现为模式识别受体(pattern recognition receptor,PRR)功能,如结核分枝杆菌和人类免疫缺陷病毒(human immunodeficiency virus,HIV)与DC-SIGN相互作用后,可通过激活核因子κB(nuclear factor κB,NF-κB)产生免疫应答分子白细胞介素10(interleukin 10,IL-10)[4]。病毒进入细胞复制是诱发细胞应答的关键,NF-κB激活还需病原体同时激活Toll样受体(Toll-like receptor,TLR)[4-5]。
鼠肝炎病毒(mouse hepatitis virus,MHV)是乙型冠状病毒(beta coronavirus)原型种,可导致小鼠肝炎和中枢神经性病变,是研究冠状病毒分子生物学和致病性的经典模式病毒[6-7]。鼠癌胚抗原相关细胞黏附分子1(mouse carcinoembryonic antigen-related cell adhesion molecule 1,mCEACAM1)是MHV的主要受体[8]。mCEACAM1是典型的细胞黏附分子,其膜内区包括蛋白激酶C(protein kinase C,PKC)和免疫受体酪氨酸抑制基序(immunoreceptor tyrosine-based inhibitory motif,ITIM)等,ITIM通过激活SHP1/2信号通路参与免疫耐受机制[9]。
人DC-SIGN能促进动物冠状病毒感染,如猫传染性腹膜炎病毒(feline infectious peritonitis virus,FIPV)和禽传染性支气管炎病毒(infectious bronchitis virus,IBV)感染[10-11]。但没有证据表明人DC-SIGN能介导鼠冠状病毒感染异源细胞[6-7]。本研究比较了稳定表达DC-SIGN与L-SIGN的不同类型细胞中鼠冠状病毒的复制水平,发现L-SIGN对鼠冠状病毒复制的抑制作用较DC-SIGN更明显,这主要与两者胞内区序列差异有关。DC-SIGN调节鼠冠状病毒复制的作用依赖病毒受体mCEACAM1a的存在,不能介导病毒感染无受体表达的细胞。其调控机制可能是以三聚体的形式与病毒受体相互作用,改变了病毒受体和细胞的功能,包括阻止鼠冠状病毒感染导致的细胞外信号调节激酶(extracellular signal-regulated kinase,ERK)途径信号转导下调等。
1 材料与方法 1.1 材料小鼠脑神经瘤细胞Neuro-2a购自中国科学院典型培养物保藏委员会细胞库;小鼠成纤维细胞NIH/3T3购自美国典型微生物菌种保藏中心(American Type Culture Collection,ATCC);人神经瘤细胞SH-SY5Y来自复旦大学脑科学研究院黄芳博士实验室;大鼠胚胎肺细胞L2来自美国纽约州卫生部沃兹沃思研究中心Dr. Paul Masters实验室。鼠肝炎病毒(MHV-A59)来自Dr. Paul Masters实验室[12],猪传染性胃肠炎病毒(porcine transmissible gastroenteritis virus,TGEV)(Purdue)购自ATCC。真核表达质粒pcDNA3.1/myc-HisA(Invitrogen V385-20)由本实验室保存,含人DC-SIGN、L-SIGN基因的cDNA质粒来自美国马里兰州弗雷德里克国家卫生研究院Dr. Wu实验室[13]。感受态DH5α和Top10细胞购自天根生化科技(北京)有限公司。抗鼠冠状病毒鼠多克隆抗体(pAb-MHV)由本实验室制备[14];鼠抗人DC-SIGN(CD209)、L-SIGN(CD209L)抗体购自BD PharMingen;兔抗Raf-1、丝裂原活化的细胞外信号调节激酶(mitogen-activated extracellular signal-regulated kinase,MEK)、ERK及磷酸化抗体购自Cell Signaling Technology公司;β-actin和GADPH购自BD公司;抗鼠和抗兔二抗购自GE医疗生命科学。
1.2 方法 1.2.1 细胞培养与病毒感染Neuro-2a、SH-SY5Y、NIH/3T3及NIH/3T3_vR细胞用含10%胎牛血清(fetal bovine serum,FBS)(Gibco公司)的高糖DMEM(Hyclone公司)于37 ℃、5% CO2传代培养,传代间隔3~4 d。将病毒感染用细胞培养36~48 h,密度达60%~80%,根据不同实验需要,调整病毒感染复数(multiplicity of infection,MOI)为1~10,感染1 h后更换为新鲜完全培养基,感染计时从加入病毒开始。
1.2.2 流式细胞术细胞于T25培养瓶中培养48 h,密度约为80%,用0.25%胰酶消化细胞,完全培养基终止消化。转移细胞悬液至15 mL离心管,1 000 r/min离心8 min,用磷酸盐缓冲液(phosphate buffered saline,PBS)洗细胞2次。250 μL PBS〔含1%牛血清白蛋白(bovine serum albumin,BSA)〕重悬细胞,分装至1.5 mL管中(每管100 μL约含1×106个细胞),2 500 r/min离心5 min,弃上清液。加入4 μg/mL CD209 mAb或CD209L mAb 100 μL,室温孵育1 h,800 μL PBS洗细胞2次。加入100 μL稀释的异硫氰酸荧光素(fluorescein isothiocyanate,FITC)-羊抗鼠IgG,4 ℃避光孵育30 min,800 μL PBS洗细胞2次。用400 μL 1%多聚甲醛重悬细胞,4 ℃避光保存。用FACSCalibur流式细胞仪(BD公司)检测,CELLQuest软件获取和分析结果。
1.2.3 病毒复制曲线测定与噬斑实验病毒感染后定时收集培养上清液和细胞裂解物,间隔时间前期为4 h,后期为8~12 h。采用噬斑实验测定上清液病毒滴度,蛋白免疫印迹法检测细胞中病毒S或N蛋白。将鼠L2细胞铺至D60培养皿中,37 ℃、5% CO2培养36~48 h,使密度达80%。样品用含2% FBS的1×DMEM进行10倍系列稀释至10-6。每个培养皿加入1 mL稀释上清液,37 ℃吸附2 h;等量混合60 ℃ 1.95%琼脂和37 ℃含10% FBS的2×DMEM,并维持50 ℃左右备用;吸去培养皿中的病毒液,加7 mL上述混合琼脂,凝固后置37 ℃、5% CO2培养40 h。将中性红染液3 mL加至培养皿,37 ℃染色8 h后计数。
1.2.4 人DC-SIGN和L-SIGN及融合突变体重组真核表达质粒构建分子克隆按常规方法进行[15],试剂来自NEB和TaKaRa公司。pcDNA3_DC-SIGN和pcDNA3_L-SIGN通过聚合酶链反应(polymerase chain reaction,PCR)扩增基因片段(引物T7:5′-AATACGACTCACTATAG-3′/BGH-R: 5′-TAGAAGGCACAGTCGAGG-3′),分别经Bam H Ⅰ和Eco R Ⅴ位点插入pcDNA3.1/myc-HisA。pcDNA3_LYdc通过二轮PCR将DC-SIGN N端膜内区片段(引物T7/SIGN-2R:5′-CCATGGCCAAGACACCCTG-3′)和L-SIGN C端跨膜区-颈区-糖类识别区(carbohydrate recognition domain,CRD)片段(引物SIGN-1F:5′-CAGGGTGTCTTGGCCATGG-3′/BGH-R)融合(引物T7/BGH-R);pcDNA3_LCdc通过引物将DC-SIGN C端糖类结合区末端16个氨基酸加至L-SIGN C端(引物T7/SIGN-4R:5′-TGGATA-TCTGCAGAATTCTACGCAGGAGGGGGGTT-TGGGGTGGCAGGGGCTGGAGAAAGAAAC-TGTTCTTCGTCTCTGAAGCAGGC-3′),融合片段经Bam H Ⅰ和Eco R Ⅴ位点插入pcDNA3.1/myc-HisA。
1.2.5 稳定表达细胞系建立细胞培养于6孔板,达70%~80%密度时更换为无血清Opti MEM(Gibco公司),继续培养2 h。取5 μL(2.5~5 μg)质粒加至100 μL无血清Opti MEM,另取5 μL Lipofectamine 2000(Invitrogen公司)加至100 μL无血清Opti MEM,将两者轻轻混匀,室温静置5 min后加至培养板,37 ℃、5% CO2培养6 h,培养液换为10% FBS-DMEM。每个样品转染两个培养孔,一孔于48 h收集细胞裂解液检测蛋白表达情况,另一孔于72 h更换为含500~1 000 ng/μL G418(Invitrogen公司)的10% FBS-DMEM维持培养。1周后转入T25培养瓶,每周更换2~3次培养基和1次培养瓶,3~4周后转入T75培养瓶扩大培养并冻存。
1.2.6 RNA提取及反转录PCR吸去D60培养皿或T25培养瓶中培养基,加入1 mL TRIzol。室温静置5 min后转移至离心管中,加0.2 mL氯仿并充分混匀,4 ℃、12 000 r/min离心5 min。将上层水相转移至新离心管中,加入0.5 mL异丙醇,充分混匀于-20 ℃静置2 h以上。4 ℃、12 000 r/min离心10 min,弃上清液,沉淀用70%乙醇洗2次,室温晾干。用25~50 μL无菌水(含1 U/μL RNase Inhibitor)溶解RNA,-80 ℃保存。cDNA合成:取10 μL RNA与2 μL Oligo-dT(10 mmol/L)混匀,80 ℃孵育10 min,冷却至室温;加入4 μL 5×M-MLV Buffer、2 μL 10 mmol/L dNTP、1 μL RNase Inhibitor(40 U/μL)、1 μL M-MLV(200 U/μL),42 ℃孵育1 h,70 ℃孵育15 min,冰上冷却。PCR:混合32 μL ddH2O、10 μL 5×PCR Buffer、1 μL dNTP(10 mmol/L)、0.5 μL Taq酶,加入2 μL特异性引物和5 μL cDNA,94 ℃变性5 min,30个循环(94 ℃ 30 s、52 ℃ 45 s、72 ℃ 60 s),72 ℃延伸5 min。产物用1%琼脂糖电泳检测。
1.2.7 蛋白免疫印迹法蛋白电泳及转印实验用Bio-Rad Mini装置完成。样本缓冲液(Tris/Glycine/SDS)购自CalBioChem公司。细胞裂解液样品加等体积2×样品缓冲液,100 ℃、5 min变性处理。配制丙烯酰胺(Amresco公司)凝胶(浓缩胶5%,分离胶8%~12%),15 mA恒流电泳60~90 min。蛋白相对分子质量采用预染PageRuler标准。分离胶中蛋白在4 ℃、250 mA条件下经1~3 h转移至聚偏氟乙烯(polyvinylidene fluoride,PVDF)膜(Bio-Rad公司)。PVDF膜用5%脱脂奶粉封闭,与一定稀释度的一抗4 ℃孵育过夜,洗涤后与二抗室温孵育1 h,采用增强化学发光法(enhanced chemiluminescence,ECL)(Bio-Rad公司)发光,X线片显影。
1.2.8 免疫荧光染色将传代细胞铺至玻璃底小型细胞培养皿(In Vitro Scientific公司),培养24~48 h至细胞密度为60%~80%,用MOI=10.0的病毒感染至特定时间,收集培养液。单层细胞用冷PBS洗3次,4%多聚甲醛(Sigma公司)室温固定30 min,5% BSA封闭1 h,4 ℃保存。一抗4 ℃孵育过夜,洗涤后二抗室温孵育1 h,与4′, 6-二脒基-2-苯基吲哚(4′, 6-diamidino-2-phenylindole,DAPI)孵育30 min,晾干封片。用Leica TCS SP8激光共聚焦显微镜观察结果。
2 结果 2.1 DC-SIGN和L-SIGN过表达抑制鼠冠状病毒复制为观察DC-SIGN和L-SIGN对鼠冠状病毒复制的影响,将构建的真核重组质粒转染至NIH/3T3细胞,经筛选、传代培养、流式细胞检测分析,获得能稳定表达人DC-SIGN和L-SIGN的NIH/3T3细胞系(图 1A)。鼠冠状病毒(MHV-A59)感染稳定表达DC-SIGN或L-SIGN的细胞系。病毒测定结果表明,DC-SIGN和L-SIGN过表达均可降低MHV-A59滴度(图 1B);病毒S和N蛋白表达水平降低并滞后(图 1C)。L-SIGN对MHV-A59复制和蛋白表达的抑制作用比DC-SIGN更显著,可能与DC-SIGN和L-SIGN之间的差异有关(图 1C)。
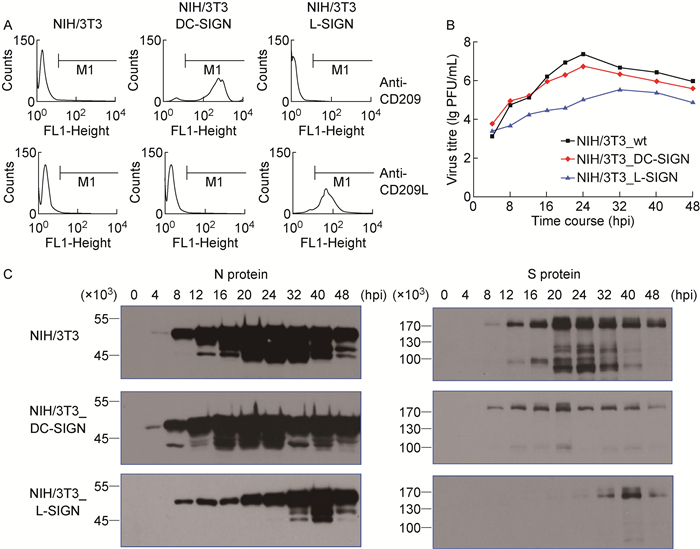
|
| A: DC-SIGN or L-SIGN expressed on the membrane of murine NIH/3T3 cells stably transfected with eukaryotic expression plasmids were detected by flow cytometry. B: Replication of murine coronavirus in murine NIH/3T3 cells stably transfected with DC-SIGN or L-SIGN plasmids. Viruses in supernatants were titrated at 4 to 48 h postinfection (hpi) by plaque assay (titer shown in lg PFU/mL). C: Murine coronavirus N and S proteins in murine NIH/3T3 cells stably transfected with DC-SIGN or L-SIGN plasmids were analyzed by Western blotting. 图 1 DC-SIGN和L-SIGN对鼠冠状病毒在NIH/3T3细胞中复制的影响 Fig. 1 Effects of DC-SIGN and L-SIGN on replication of murine coronavirus in NIH/3T3 cells |
DC-SIGN与L-SIGN的CRD同源,但两者分布在不同类型细胞对病毒感染的调节存在差异[16-17]。比较一级结构可看出,两者N端胞内区均包含双亮氨酸和三酸性氨基酸簇(LL/EED),胞外区C端的CRD同源性为84%,但L-SIGN胞内区序列较长,无YxxL酪氨酸激酶信号,末端缺少16个富含脯氨酸的序列(图 2A)。为此,分别将DC-SIGN膜内区序列和膜外区C端16个氨基酸同源替换或添加至L-SIGN,构建两个嵌合突变体LYdc和LCdc(图 2A)。
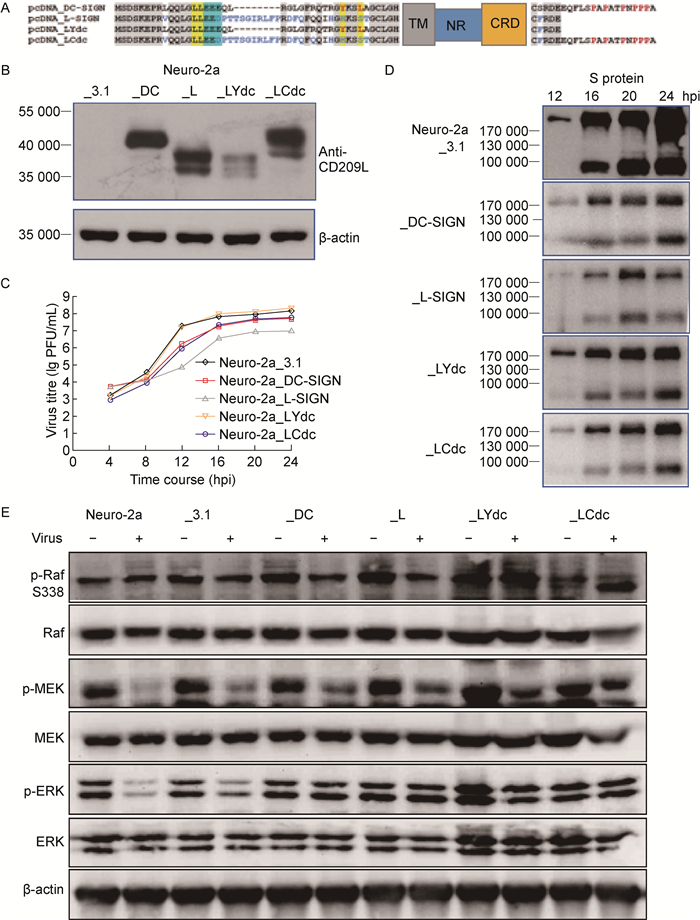
|
| A: Diagram shows partial amino acid sequences and functional domains of DC-SIGN and L-SIGN. Signal motifs in N-terminal endodomain and C-terminal 21 or 5 residues of carbohydrate recognition domain (CRD) are shown. The transmembrane (TM) and neck regions (NRs) are the representive of rectangles. Chimeric L-SIGN_Ydc and _Cdc were constructed by grafting YxxL and C-terminal 16-peptide from DC-SIGN into L-SIGN respectively. B: Expression levels were analyzed by Western blotting in murine Neuro-2a cells stably transfected with DC-SIGN, L-SIGN, chimeric L-SIGN_Ydc or _Cdc plasmids. C: Titers of murine coronavirus in murine Neuro-2a cells stably transfected with above plasmids were titrated (lg PFU/mL of supernatant) at 4, 8, 12, 16, 20, 24 hpi. D: Viral S protein in the cell lysates was measured by Western blotting at 12, 16, 20, 24 hpi. E: Regulation of MAPK pathway at early infection of murine coronavirus. Neuro-2a cells stably expressing DC-SIGN, L-SIGN, chimeric L-SIGN_Ydc or _Cdc were infected with 2.0 MOI of murine coronavirus (MHV-A59) for 1 h. Total and phosphorylated (p-) Raf, MEK, and ERK were analyzed by Western blotting. β-actin is shown as a loading control. 图 2 DC-SIGN N端和C端氨基酸在鼠冠状病毒复制调控中的作用 Fig. 2 Roles of N- and C-terminal residues of DC-SIGN in replication of murine coronavirus |
将pcDNA3_DC-SIGN、pcDNA3_L-SIGN、pcDNA3_LYdc、pcDNA3_LCdc质粒分别转染鼠脑神经瘤细胞Neuro-2a,建立稳定表达相应蛋白的细胞系(图 2B)。在这些Neuro-2a细胞系中,DC-SIGN和L-SIGN均能抑制MHV-A59复制,L-SIGN的抑制作用较DC-SIGN更显著(图 2C)。而同源替换DC-SIGN的功能域显著减弱了L-SIGN对病毒复制的抑制作用,LYdc比LCdc显著。在过表达LYdc的细胞系中,病毒滴度恢复至对照细胞水平(图 2C)。病毒S蛋白的表达水平与病毒滴度变化趋势基本一致(图 2D)。
鼠冠状病毒感染导致Neuro-2a细胞信号通路分子MEK和ERK磷酸化水平显著下降,Raf-1磷酸化水平无明显影响(图 2E)。鼠冠状病毒感染过表达DC-SIGN、L-SIGN及其嵌合蛋白LYdc和LCdc的Neuro-2a细胞系中,ERK和Raf-1磷酸化水平不受影响,MEK磷酸化水平显著下降,但下降程度低于未转染细胞(图 2E)。
2.3 DC-SIGN和L-SIGN抑制鼠冠状病毒复制依赖病毒受体的存在鼠冠状病毒可感染表达病毒受体mCEACAM1a的NIH/3T3和Neuro-2a细胞(图 1~2)。为研究DC-SIGN调节鼠冠状病毒复制与受体mCEACAM1a的关系,将NIH/3T3于39 ℃反复传代,获得不能转录mCEACAM1a mRNA的缺陷细胞株(NIH/3T3_vR),MHV-A59在其内的复制水平显著降低(图 3A)。
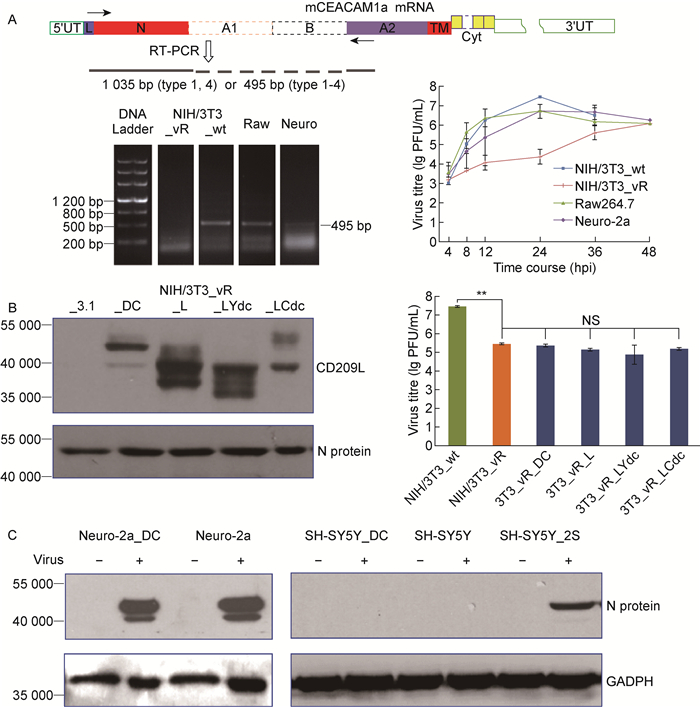
|
| A: RT-PCR of mCEACAM1a and murine coronavirus replication in receptor-deficient NIH/3T3 (NIH/3T3_vR), wild-type NIH/3T3 (NIH/3T3_wt), Raw264.7 and Neuro-2a cells. Diagram shows the functional domains of polypeptide and mRNA of mCEACAM1a. Amplification primers and product size were marked. B: DC-SIGN, L-SIGN, chimeric L-SIGN_Ydc and _Cdc plasmids were transfected in receptor-deficient NIH/3T3 cells. N protein and titres of murine coronavirus at 24 hpi were analyzed by Western blotting and plaque assay respectively. **P < 0.01. NS: no significant difference. C: N protein of murine coronavirus was detected in Neuro-2a cells expressing DC-SIGN, SH-SY5Y cells expressing mCEACAM1a-2S or DC-SIGN at 24 hpi by Western blotting. 图 3 DC-SIGN对鼠冠状病毒复制的调控依赖病毒受体分子mCEACAM1a Fig. 3 Regulation of DC-SIGN on murine coronavirus replication is dependent on viral receptor mCEACAM1a |
将pcDNA3_DC-SIGN、pcDNA3_L-SIGN、pcDNA3_LYdc、pcDNA3_LCdc分别转染缺陷细胞株NIH/3T3_vR,能稳定表达4种蛋白(图 3B);MHV-A59在4种转染细胞中及不表达重组蛋白的NIH/3T3_vR细胞中病毒滴度无显著差异(图 3B)。MHV-A59在稳定表达DC-SIGN但不表达病毒受体mCEACAM1a的人神经母细胞瘤细胞SH-SY5Y中不能复制,感染细胞中检测不到病毒N蛋白;而表达DC-SIGN的鼠Neuro-2a细胞中N蛋白表达水平显著下降(图 3C)。结果提示,DC-SIGN和L-SIGN对病毒复制的影响依赖病毒受体mCEACAM1a的表达。
2.4 DC-SIGN与病毒受体相互作用为分析细胞表面DC-SIGN与mCEACAM1a可能存在的相互作用,用MHV-A59感染稳定表达DC-SIGN的NIH/3T3细胞,免疫荧光法检测细胞表面的DC-SIGN和mCEACAM1a。结果显示,随MHV-A59孵育时间延长,DC-SIGN和mCEACAM1a共定位更明显,而在无病毒感染细胞中两者共定位不明显(图 4A)。蛋白免疫印迹检测结果表明,MHV-A59感染稳定表达DC-SIGN的Neuro-2a细胞可导致受体mCEACAM1a减少,蛋白交联剂DTSSP处理后mCEACAM1a L亚型增加(图 4B),而同样处理的NIH/3T3细胞无增加现象(图 4B),与两种细胞的mCEACAM1a异型分布差异一致。

|
| A: Co-location of DC-SIGN and mCEACAM1a in NIH/3T3 cells infected with murine coronavirus. DC-SIGN is stained with AF-488 (green) and mCEACAM1a is stained with PE (red). Confocal photography was conducted at 15 and 60 min after virus infection, at 30 min after mock infection. Bar is 50 μm. The insets show an enlarged cell. B:Co-expression of DC-SIGN and mCEACAM1a in Neuro-2a and NIH/3T3 cells. Cells stably transfected with DC-SIGN plasmid were infected with 10 MOI of murine coronavirus MHV-A59 or porcine coronavirus TGEV for 30 min and then cells were treated with 10 μmol DTSSP for 15 min. 图 4 DC-SIGN与鼠冠状病毒受体mCEACAM1a相互作用 Fig. 4 Interaction of DC-SIGN with murine coronavirus receptor mCEACAM1a |
蛋白交联剂处理后,DC-SIGN形成明显的三聚体和四聚体(图 4B)。与非同源TGEV感染细胞和无病毒感染细胞比较,MHV-A59感染导致DC-SIGN三聚体减少,而DC-SIGN四聚体及单体与mCEACAM1a异源二聚体均轻度增加(图 4B)。结果提示,鼠冠状病毒既可能直接激活DC-SIGN,也可能同时结合DC-SIGN与mCEACAM1a,促使两者相互作用,协同调控病毒在细胞中的复制。
3 讨论人源CLR在冠状病毒感染过程中的作用多样而复杂,可直接促进病毒进入细胞提高复制水平,也能诱导细胞产生抗病毒应答。如L-SIGN可作为严重急性呼吸综合征冠状病毒(severe acute respiratory syndrome coronavirus,SARS-CoV)的受体介导病毒感染,也可表现为免疫保护作用[18-20]。人DC-SIGN和L-SIGN能促进人及异源的猫和禽冠状病毒感染[10-11]。本研究发现,在表达人DC-SIGN和L-SIGN的鼠细胞中,鼠冠状病毒的复制受到抑制(图 1)。在不表达mCEACAM1a的人神经细胞中,即使表达DC-SIGN也不能介导病毒感染(图 3)。DC-SIGN可能独立或通过与病毒受体相互作用,拮抗病毒感染导致的ERK通路下调,引起鼠冠状病毒复制降低(图 2、图 4)。
DC-SIGN主要由DC表达,L-SIGN主要由肝窦或淋巴结内皮细胞表达,前者通过内体形成促进病毒进入细胞,而后者主要表现为免疫调节作用,两者在功能上的差异与来源细胞和氨基酸序列的不同有关[5, 16, 21]。两者较短的N端膜内区中存在的保守双亮氨酸和三酸性氨基酸簇(LL/DDD)为内化所必需,有助于介导病毒进入细胞[17]。DC-SIGN膜内区含有YxxL基序,而L-SIGN无此基序,膜内区更长(图 2A)。同源序列替换证明,DC-SIGN胞内区YxxL信号有消除L-SIGN抑制病毒复制的作用,但CRD末端16个氨基酸不明显(图 2)。膜内YxxL基序对识别真菌和寄生虫抗原CLR Dectin-1和CLEC-2信号是必需的,但在DC-SIGN中作用不明显[17, 22]。本研究发现,鼠冠状病毒感染后,Raf-1磷酸化(S338)变化不明显,但MEK和ERK磷酸化水平明显下调,DC-SIGN、L-SIGN及其N端和C端序列嵌合体均能拮抗这种下调作用(图 2E)。大部分病毒及其膜蛋白通过DC-SIGN和L-SIGN激活丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)通路信号分子[23-24],而病毒受体本身具有的信号激活功能作用可能相反。
病原体本身通过DC-SIGN激活Raf-1磷酸化依赖激酶Src和Pak,进入细胞后通过其他组分激活TLR,最终激活NF-κB产生免疫调节作用[4-5]。但与DC不同,鼠Neuro-2a和NIH/3T3细胞不具有免疫细胞功能,导致DC-SIGN作用不明显,而上皮细胞来源的L-SIGN对病毒复制发挥主导调控作用。
鼠冠状病毒特异性受体mCEACAM1是典型黏附分子,通过识别细胞自身的糖蛋白如癌胚抗原(carcinoembryonic antigen,CEA)、IgFc、细胞间黏附分子2(intercellular adhesion molecule 2,ICAM-2)和ICAM-3等参与免疫调节与肿瘤发生[9]。不同组织来源细胞的mCEACAM1有不同RNA拼接异型,N端膜外区结构域异型2[1, 4]和4[1-4]可能导致不同鼠细胞对病毒的易感性不同(图 3A)。mCEACAM1a C端膜内区结构域异型S和L所含的信号激活序列不同,L和S型均有PKC激活信号,但只有L型含有重复的ITIM信号[9]。Neuro-2a细胞同时表达S和L两种胞内区亚型,病毒感染导致PKC磷酸化上调,而有DC-SIGN存在时PKC下调(数据未列)。鼠冠状病毒感染后PKC如何下调MAPK通路信号分子,以及ITIM信号中YxxL与DC-SIGN膜内区YxxL在激活受体酪氨酸激酶(receptor tyrosine kinase,RTK)信号通路中的差异仍有待进一步阐明。此外,NIH/3T3于39 ℃反复传代后产生的mCEACAM1a表达缺陷细胞株(NIH/3T3_vR)仍然支持MHV-A59在其内复制,但病毒滴度和N蛋白表达明显降低(图 3A、图 3B)。推测当mCEACAM1a表达减少或缺陷时,细胞中其他黏附分子代偿性增加,而这些糖蛋白结构功能域与鼠冠状病毒结合的能力增强,也不排除NIH/3T3细胞存在鼠冠状病毒的辅助受体。
DC-SIGN和L-SIGN的CRD识别含甘露聚糖或果糖寡聚糖结构的配体分子,能直接与SARS-CoV S蛋白相互作用介导病毒进入细胞[25-26]。本研究发现,DC-SIGN不能介导鼠冠状病毒进入细胞,病毒粒子更有可能发挥配体的作用,直接与CLR结合并激活MAPK信号分子(图 3)。此外,鼠冠状病毒可能同时结合DC-SIGN和mCEACAM1a,促进两者靠近发生相互作用,从而抑制mCEACAM1a介导病毒进入或改变其诱导的信号通路(图 4)。
鼠冠状病毒感染可引发肝脏和中枢神经系统免疫病理[6-7]。在小鼠中存在人DC-SIGN和L-SIGN同源基因簇,表达7~8种同源分子,称为mSIGNR1~8[27]。但这些分子在小鼠组织细胞中的表达不恒定,且呈功能多样性,导致建立DC-SIGN敲除小鼠模型困难,从而限制了DC-SIGN的深入研究[28]。本研究构建了稳定表达人DC-SIGN和L-SIGN的NIH/3T3和Neuro-2a细胞系,探讨了鼠冠状病毒复制调控的相关机制,有助于加深对冠状病毒感染与致病机制的理解。
| [1] |
van Breedam W, Pohlmann S, Favoreel HW, de Groot RJ, Nauwynck HJ. Bitter-sweet symphony: glycan-lectin interactions in virus biology[J]. FEMS Microbiol Rev, 2014, 38(4): 598-632.
[DOI]
|
| [2] |
Pokidysheva E, Zhang Y, Battisti AJ, Bator-Kelly CM, Chipman PR, Xiao C, Gregorio GG, Hendrickson WA, Kuhn RJ, Rossmann MG. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN[J]. Cell, 2006, 124(3): 485-493.
[DOI]
|
| [3] |
Cormier EG, Durso RJ, Tsamis F, Boussemart L, Manix C, Olson WC, Gardner JP, Dragic T. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus[J]. Proc Natl Acad Sci USA, 2004, 101(39): 14067-14072.
[DOI]
|
| [4] |
Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB[J]. Immunity, 2007, 26(5): 605-616.
[DOI]
|
| [5] |
Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis[J]. Annu Rev Immunol, 2012, 30: 491-529.
[DOI]
|
| [6] |
Masters PS, Perlman S. Coronaviridae [M]. In: Knipe DM, Howley PM. eds. Fields virology. 6th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins, 2013: 825-854.
|
| [7] |
Weiss SR, Leibowitz JL. Coronavirus pathogenesis[J]. Adv Virus Res, 2011, 81: 85-164.
[DOI]
|
| [8] |
Williams RK, Jiang GS, Holmes KV. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins[J]. Proc Natl Acad Sci USA, 1991, 88(13): 5533-5536.
[DOI]
|
| [9] |
Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis[J]. Cancer Metastasis Rev, 2013, 32(3-4): 643-671.
[DOI]
|
| [10] |
Regan AD, Whittaker GR. Utilization of DC-SIGN for entry of feline coronaviruses into host cells[J]. J Virol, 2008, 82(23): 11992-11996.
[DOI]
|
| [11] |
Zhang Y, Buckles E, Whittaker GR. Expression of the C-type lectins DC-SIGN or L-SIGN alters host cell susceptibility for the avian coronavirus, infectious bronchitis virus[J]. Vet Microbiol, 2012, 157(3-4): 285-293.
[DOI]
|
| [12] |
Kuo L, Godeke GJ, Raamsman MJ, Masters PS, Rottier PJ. Retargeting of coronavirus by substitution of the spike glycoprotein ectodomain: crossing the host cell species barrier[J]. J Virol, 2000, 74(3): 1393-1406.
[DOI]
|
| [13] |
Wu L, Martin TD, Vazeux R, Unutmaz D, KewalRamani VN. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission[J]. J Virol, 2002, 76(12): 5905-5914.
[DOI]
|
| [14] |
郭佳慧, 文荣, 王玉燕, 张浩旸, 刘红, 徐娅佳, 叶荣. 鼠冠状病毒核衣壳蛋白的抗体制备与抗原决定簇分析[J]. 微生物与感染, 2017, 12(2): 79-88. [URI]
|
| [15] |
Green MR, Sambrook J. Molecular cloning: A laboratory manual[M]. 4th ed. New York: Cold Spring Harbor Laboratory Press, 2012.
|
| [16] |
Koppel EA, van Gisbergen KP, Geijtenbeek TB, van Kooyk Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation[J]. Cell Microbiol, 2005, 7(2): 157-165.
[PMC]
|
| [17] |
Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pohlmann S, Suzuki-Inoue K, Ozaki Y, Watson SP, Pearce AC. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YxxL-dependent signaling cascade[J]. J Biol Chem, 2007, 282(17): 12397-12409.
[DOI]
|
| [18] |
Jeffers SA, Tusell SM, Gillim-Ross L, Hemmila EM, Achenbach JE, Babcock GJ, Thomas WD Jr, Thackray LB, Young MD, Mason RJ, Ambrosino DM, Wentworth DE, Demartini JC, Holmes KV. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus[J]. Proc Natl Acad Sci USA, 2004, 101(44): 15748-15753.
[DOI]
|
| [19] |
Chan VS, Chan KY, Chen Y, Poon LL, Cheung AN, Zheng B, Chan KH, Mak W, Ngan HY, Xu X, Screaton G, Tam PK, Austyn JM, Chan LC, Yip SP, Peiris M, Khoo US, Lin CL. Homozygous L-SIGN (CLEC4M) plays a protective role in SARS coronavirus infection[J]. Nat Genet, 2006, 38(1): 38-46.
[DOI]
|
| [20] |
Jeffers SA, Hemmila EM, Holmes KV. Human coronavirus 229E can use CD209L (L-SIGN) to enter cells[J]. Adv Exp Med Biol, 2006, 581: 265-269.
[DOI]
|
| [21] |
Conde P, Rodriguez M, van der Touw W, Burns M, Miller J, Brahmachary M, Chen HM, Boros P, Rausell-Palamos F, Yun TJ, Riquelme P, Rastrojo A, Aguado B, Stein-Streilein J, Tanaka M, Zhou L, Zhang J, Lowary TL, Ginhoux F, Park CG, Cheong C, Brody J, Shannon J, Turley SJ, Lira SA, Bronte V, Gordon S, Heeger PS, Merad M, Hutchinson J, Chen SH, Ochando J. DC-SIGN+ macrophages control the induction of transplantation tolerance[J]. Immunity, 2015, 42(6): 1143-1158.
[DOI]
|
| [22] |
Zhu LL, Zhao XQ, Jiang CY, You Y, Chen XP, Jiang YY, Jia XM, Lin X. C-type lectin receptors dectin-3 and dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection[J]. Immunity, 2013, 39(2): 324-334.
[DOI]
|
| [23] |
Johnson TR, McLellan JS, Graham BS. Respiratory syncytial virus glycoprotein G interacts with DC-SIGN and L-SIGN to activate ERK1 and ERK2[J]. J Virol, 2012, 86(3): 1339-1347.
[DOI]
|
| [24] |
Zhao LJ, Wang W, Ren H, Qi ZT. ERK signaling is triggered by hepatitis C virus E2 protein through DC-SIGN[J]. Cell Stress Chaperones, 2013, 18(4): 495-501.
[DOI]
|
| [25] |
Yang ZY, Huang Y, Ganesh L, Leung K, Kong WP, Schwartz O, Subbarao K, Nabel GJ. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN[J]. J Virol, 2004, 78(11): 5642-5650.
[DOI]
|
| [26] |
Han DP, Lohani M, Cho MW. Specific asparagine-linked glycosylation sites are critical for DC-SIGN- and L-SIGN-mediated severe acute respiratory syndrome coronavirus entry[J]. J Virol, 2007, 81(21): 12029-12039.
[DOI]
|
| [27] |
Powlesland AS, Ward EM, Sadhu SK, Guo Y, Taylor ME, Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins[J]. J Biol Chem, 2006, 281(29): 20440-20449.
[DOI]
|
| [28] |
Garcia-Vallejo JJ, van Kooyk Y. The physiological role of DC-SIGN: a tale of mice and men[J]. Trends Immunol, 2013, 34(10): 482-486.
[DOI]
|
 2018, Vol. 13
2018, Vol. 13


