肠道病毒71型(enterovirus type 71,EV71)为单股正链RNA病毒,基因组约为7.4 kb,只含有1个开放读码框(open reading frame,ORF),编码1个长链多聚蛋白[1]。EV71感染常引起婴幼儿手足口病(hand,foot and mouth disease,HFMD),还可引起中枢神经系统并发症等重症,甚至导致死亡[2]。目前研究认为,EV71诱发重症的原因主要与病毒感染诱导细胞程序性死亡(programmed cell death,PCD)及诱导细胞产生大量炎症因子有关[1, 3]。细胞程序性死亡在宿主防御外来病原体感染及激活免疫应答中有重要作用。病毒感染可通过激活不同的信号通路触发细胞程序性死亡,且不同信号通路常发生交叉作用,形成特征性细胞死亡形式,主要包括细胞凋亡(apoptosis)、细胞焦亡(pyroptosis)和细胞坏死性凋亡(necroptosis)[4]。
细胞凋亡是非裂解性细胞程序性死亡,含半胱氨酸的天冬氨酸蛋白水解酶(cysteinyl aspartate specific proteinase,caspase)在此过程中处于中心调控环节,通常包括线粒体介导的内源性途径和肿瘤坏死因子受体(tumour necrosis factor receptor,TNF-R)、Fas配体(Fas ligand,FasL)等介导的外源性途径[5-6]。裂解性细胞程序性死亡通常伴随细胞膜过度膨胀崩解的现象,主要包括细胞焦亡和坏死性凋亡。细胞焦亡信号通路可由caspase介导,包括caspase-1/4/5/11等,它们又称为炎性caspase,可对下游效应蛋白Gasdermin D进行切割产生有活性的N端短片段,形成多聚体并在质膜形成孔道,迫使细胞膜裂解,进而诱导细胞裂解性死亡[7-8]。坏死性凋亡是一种不依赖caspase活性的细胞死亡方式,通常由活化的受体相互作用蛋白激酶1(receptor-interacting protein kinase 1,RIPK1)和RIPK3促使MLKL(mixed lineage kinase domain-like protein)磷酸化进行信号转导,磷酸化的MLKL组成同源复合物,在细胞膜形成孔洞,介导损伤相关分子模式(damage-associated molecular pattern,DAMP)释放,进而导致炎症细胞募集至被感染部位[7, 9-10]。
由于细胞焦亡和坏死性凋亡信号通路均伴随炎症因子产生[11],EV71感染后也可引起大量炎症因子产生[1],据此推测EV71感染可能与细胞焦亡和坏死性凋亡有关。本研究通过检测EV71感染后细胞程序性死亡相关指标,发现EV71感染可诱导细胞程序性死亡,且以细胞凋亡为主,或可能诱导细胞焦亡和坏死性凋亡,但Gasdermin D介导的细胞焦亡和磷酸化MLKL(p-MLKL)介导的细胞坏死性凋亡信号通路似乎并未被激活。
1 材料与方法 1.1 材料EV71病毒株(GenBank登录号:HQ891927,064-Shanghai)由本实验室分离保存。横纹肌瘤细胞(rhabdomyosarcoma,RD)、人神经母细胞瘤细胞(SH-SY5Y)、子宫颈癌细胞(HeLa)、人结肠癌细胞(HT-29)购自中国科学院细胞库。EV71-VP1鼠单克隆抗体购自Abnova公司;DyLight 594荧光二抗、caspase-9(#9502)、caspase-3(#9665)、多聚ADP核糖聚合酶[poly (ADP-ribose) polymerase,PARP](#9542)、MLKL(#14993)、p-MLKL(#91689)、β-actin抗体购自Cell Signaling Technology公司; GSDMD(ab210070)抗体购自Abcam公司。JC-1线粒体膜电位检测试剂盒、Annexin V-FITC/PI凋亡检测试剂盒购自上海翊圣生物科技有限公司。乳酸脱氢酶(lactate dehydrogenase,LDH)试剂盒购自Promega公司。Z-VAD-FMK、十字孢碱(Staurosporine)均购自Med Chem Express公司。
1.2 方法 1.2.1 细胞培养和EV71感染RD、SH-SY5Y和HeLa细胞均于含10%胎牛血清(fetal bovine serum,FBS)的DMEM培养基中常规培养。HT-29细胞则用McCoy’s 5A培养基(含10% FBS)。当上述细胞生长融合至80%~90%单层时,用感染复数(multiplicity of infection,MOI)=1或10的EV71感染,2 h后更换正常培养液,于含5% CO2的37 ℃培养箱中继续培养。
1.2.2 共聚焦免疫荧光分析EV71(MOI=1)感染RD细胞6 h后,用4%多聚甲醛穿透30 min,预冷的100%甲醇固定15 min,与稀释于含5%小牛血清封闭液中的鼠EV71-VP1抗体4 ℃孵育过夜,与DyLight 594红色荧光羊抗鼠IgG抗体室温孵育2 h,与4′, 6-二脒基-2-苯基吲哚(4′, 6-diamidino-2-phenylindole,DAPI)染色液室温孵育15 min,于Leica激光共聚焦显微镜下观察拍照。
1.2.3 蛋白免疫印迹检测用含十二烷基硫酸钠(sodium dodecyl sulfate,SDS)的Loading Buffer处理细胞样品,沸水浴5 min;配制5%浓缩胶和10%分离胶,30 mA恒流电泳;冰浴,200 mA将蛋白转移至聚偏氟乙烯(polyvinylidene fluoride,PVDF)膜上,与相应靶蛋白抗体4 ℃孵育过夜;与辣根过氧化物酶(horseradish peroxidase,HRP)标记的二抗室温孵育2 h,用增强化学发光法(enhanced chemiluminescence,ECL)检测蛋白表达情况。
1.2.4 JC-1检测线粒体膜电位变化EV71(MOI=1)感染RD细胞后,分别于指定时间点加入JC-1染色工作液,在细胞培养箱中孵育20 min,经JC-1染色缓冲液清洗,于荧光显微镜下观察拍照。
1.2.5 Annexin V-FITC/PI染色检测EV71感染所致细胞程序性死亡的特征EV71(MOI=1)感染RD和SH-SY5Y细胞后,用不含乙二胺四乙酸(ethylenediamine tetraacetic acid,EDTA)的胰酶消化,200 g 4 ℃离心5 min,收集细胞;加入100 μL 1×Binding Buffer重悬细胞,加入5 μL Annexin V-FITC和10 μL PI Staining Solution,避光室温反应10~15 min;加入400 μL 1×Binding Buffer,用荧光显微镜检测。
1.2.6 LDH释放分析用泛caspase抑制剂Z-VAD-FMK(20 μmol/L)处理RD、SH-SY5Y和HeLa细胞30 min后,用EV71感染细胞(RD和SH-SY5Y细胞以MOI=1感染,HeLa细胞以MOI=10感染);在指定时间点转移50 μL病毒感染细胞的培养上清液至新的96孔板对应孔中,另加50 μL CytoTox 96? Reagent覆盖,室温避光孵育30 min;最后加50 μL Stop Solution终止反应,于490 nm处检测其吸光度。
1.3 统计学分析采用Graphpad Prism 6软件统计作图,数据以x±s表示,并进行t检验,P < 0.05为差异有统计学意义。
2 结果 2.1 EV71感染诱导细胞程序性死亡的形态和结构特征为研究EV71感染诱导细胞程序性死亡的形态学特征,首先用EV71感染RD和SH-SY5Y细胞,于感染后指定时间在光学显微镜下观测形态学变化。结果显示,EV71感染上述细胞后,均出现变圆脱落、体积变小、细胞核皱缩等凋亡特征,且少量细胞有细胞膜过度膨胀变圆,甚至裂解的现象(图 1A)。用免疫荧光和激光共聚焦显微技术观察EV71感染的RD细胞,发现EV71感染后,部分细胞染成红色,显示病毒VP1蛋白在这些细胞中表达,EV71感染细胞成功。为更好地显示病毒感染后细胞核和细胞的形态变化,同时进行组成性表达的STAT3α染色,发现感染细胞的细胞核明显皱缩,有裂解分离现象,而未感染细胞的细胞核多形态完整(图 1B)。通过JC-1染色发现,随着病毒感染时间延长,被染成绿色的细胞数量逐渐增多,提示EV71感染可诱导细胞线粒体膜逐渐崩解(图 1C)。而Annexin V-FITC/PI染色显示,病毒感染后早期凋亡细胞(Annexin V-FITC+/PI-)和晚期凋亡或坏死细胞(Annexin V-FITC+/PI+)明显增多,提示病毒感染可导致细胞膜和细胞核膜损伤(图 1D)。上述结果提示,病毒感染可诱导细胞出现明显的程序性死亡形态学特征,少数细胞出现膨胀变大等现象,提示EV71感染可能诱导多种形式的细胞程序性死亡。
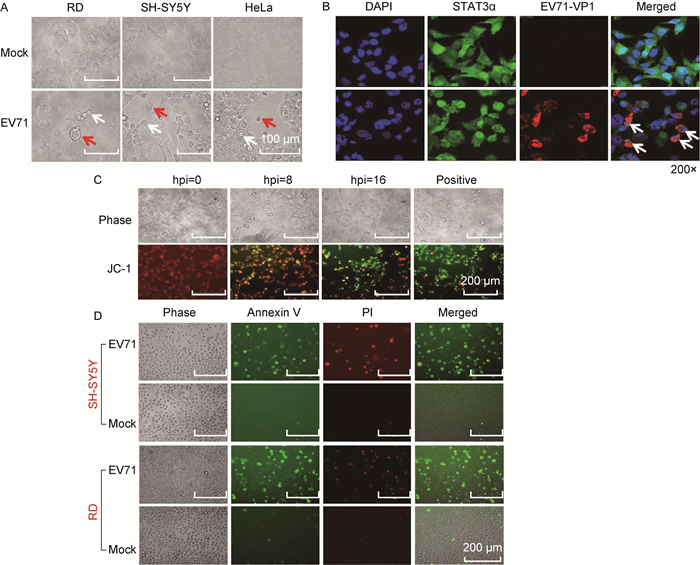
|
| A: The characteristic morphology of RD and SH-SY5Y cells infected with EV71 (MOI=1) at 16 hpi (red arrows indicate the swelling cells whereas white arrows for the shrinking cells). B: Confocal microscopy shows the abnormal nuclear morphology of the cells infected with EV71. The cells were infected with EV71 (MOI=1) at 6 hpi or mock-treated, and then undergone immunofluorescence staining with monoclonal antibodies against human STAT3α (green) and EV71-VP1 (red), respectively, and subsequently with DAPI (blue). C: JC-1 staining of RD cells infected with EV71 (MOI=1) at indicated time points. The cells treated with staurosporine at 1.0 μmol/L for 3 h were considered as positive control. D: Cell staining with PI and Annexin V-FITC. RD and SH-SY5Y cells infected with EV71 (MOI=1) for 16 h, and then the cells were trypsinized for staining. 图 1 EV71感染后细胞形态和细胞染色的特征 Fig. 1 Characteristics of cellular morphology and staining after EV71 infection |
为进一步研究EV71感染诱导细胞程序性死亡的分子生物学特征,采用蛋白免疫印迹法分析EV71感染细胞后细胞凋亡、细胞焦亡和坏死性凋亡关键调控蛋白的表达和激活情况。结果显示,细胞凋亡信号通路相关蛋白caspase-9和caspase-3被激活,细胞凋亡发生的标记蛋白PARP[12]被剪切(图 2A)。由于Gasdermin D的表达具有细胞特异性,利用表达水平较高的HeLa细胞进行分析,结果显示Gasdermin D的表达水平在病毒感染前后并没有显著变化,且未检测到被剪切激活的N端短片段(图 2B)。此外,EV71感染后,细胞坏死性凋亡关键调控蛋白MLKL的表达没有明显改变,也没有检测到p-MLKL激活(图 2B)。上述结果表明,EV71感染可诱导细胞凋亡信号通路激活,触发细胞程序性死亡,但并不明显诱导Gasdermin D和p-MLKL介导的细胞程序性死亡。
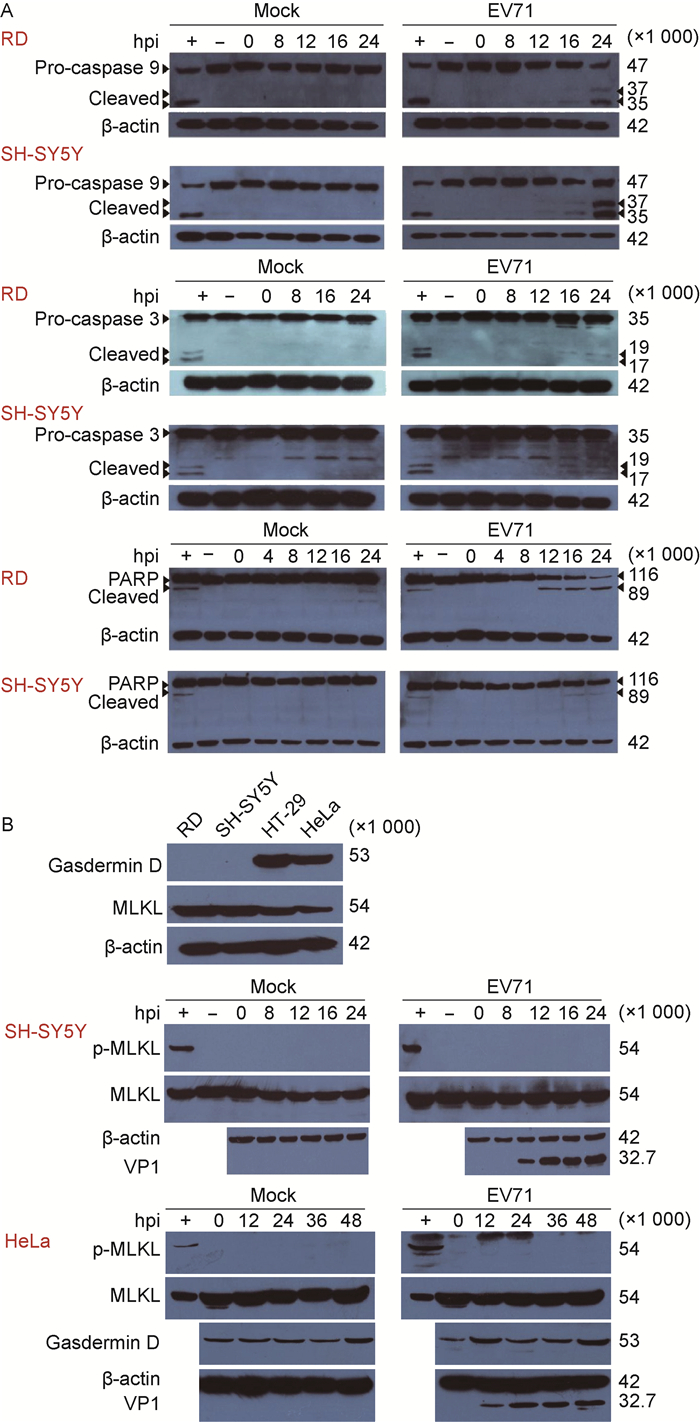
|
| A: EV71 infection induces the activation of caspases and the cleavage of PARP. The cells treated with staurosporine were set as positive control. B: EV71 infection without induction of Gasdermin D-mediated pyroptosis and p-MLKL-mediated necroptosis. The cells were infected with EV71 at MOI=1, and then collected for immunoblotting at the indicated time points. The cells treated with the mixture including Z-VAD-FMK (20 μmol/L for 30 min), human TNF-α (20 ng/mL for 7 h), SM-164 (100 nmol/L for 7 h) as positive control. + and-indicate the positive and mock-treated control, respectively. 图 2 EV71感染对细胞程序性死亡调控蛋白caspase-3、caspase-9、PARP、Gasdermin D、MLKL表达和激活的影响 Fig. 2 The expressions of programmed cell death-related proteins, including caspase-3, caspase-9, PARP, Gasdermin D and MLKL in EV71-infected cells |
EV71感染后出现多数细胞皱缩并伴有少数细胞膨胀,提示病毒感染可能诱导多种形式的细胞程序性死亡。细胞凋亡和细胞焦亡是caspase依赖的细胞程序性死亡,而坏死性凋亡是非caspase依赖的细胞程序性死亡[11],因此利用Z-VAD-FMK预处理细胞,以阻断caspase依赖的细胞凋亡和细胞焦亡,在此基础上检测病毒感染诱导细胞程序性死亡的分子生物学特征。通过流式细胞术(fluorescence-activated cell sorting,FACS)和细胞Annexin V-FITC/PI染色[13],定量EV71感染诱导的细胞程序性死亡。结果发现,细胞经Z-VAD-FMK处理后,EV71感染诱导的细胞死亡数量明显下降(图 3A和3B);蛋白免疫印迹检测结果也表明,EV71诱导细胞凋亡信号通路的关键蛋白caspase-9、PARP被激活,上述蛋白酶的剪切体水平明显下调(图 3D)。上述结果初步提示,Z-VAD-FMK处理后,caspase激活被抑制,病毒诱导的细胞程序性死亡也明显被抑制。LDH的释放被认为是细胞膜完整性的标志,也是区别细胞凋亡与其他细胞程序性死亡的重要指标[14]。分析病毒感染后细胞LDH的释放也获得类似结果:Z-VAD-FMK预处理细胞并经病毒感染后,LDH释放量随病毒感染时间延长逐渐上升,但LDH释放量明显低于未处理组(图 3C),提示EV71感染主要诱导caspase依赖的细胞程序性死亡。
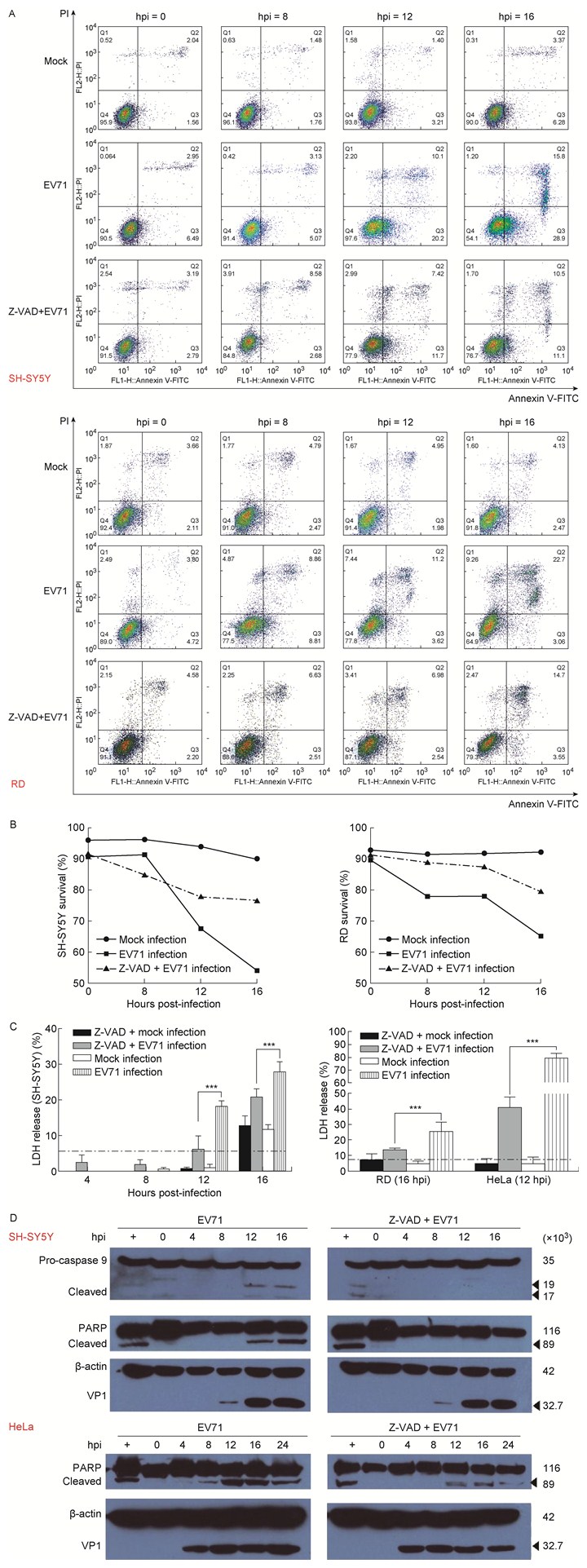
|
| A: The programmed cell death kinetics after EV71 infection identified by FACS and cell staining. The cells were pretreated with Z-VAD-FMK, a pan-caspase inhibitor, at 20 μmol/L for 1 h, or mock-treated, and then were infected with EV71 (MOI=1) at indicated time points and collected for staining with PI and Annexin V-FITC. B: Plot of the survival cells after EV71 infection as panel A. C: LDH release from EV71-induced cells was dependent on the activity of caspases. The cells were pretreated with Z-VAD-FMK or mock-treated, and then infected with EV71 as panel A. D: EV71-induced activation of caspase-9 and cleavage of PARP in a caspase-dependent manner. 图 3 EV71感染诱导细胞程序性死亡与caspase激活相关 Fig. 3 EV71-induced programmed cell death was dependent on caspase activity |
肠道病毒感染可引发严重病症,如肠道病毒感染心肌、胰腺和中枢神经系统导致的细胞程序性死亡可进一步分别诱发心肌炎、1型糖尿病和脑膜炎[15]。细胞程序性死亡包括细胞凋亡、细胞焦亡和坏死性凋亡等,在宿主抗病毒感染过程中发挥至关重要的作用[10]。EV71感染诱发的细胞程序性死亡和炎症因子风暴被认为是导致重症及死亡的原因[1, 3]。细胞凋亡是caspase介导的一种非裂解式细胞程序性死亡,细胞焦亡和坏死性凋亡通常由炎症因子介导,细胞最终以裂解方式死亡[4, 11],提示EV71诱发的重症与细胞程序性死亡存在密不可分的关系。
本研究通过对EV71感染细胞后形态学特征的观察,发现病毒感染可诱导细胞出现明显变圆脱落、体积变小及细胞核皱缩等凋亡特征,同时少量细胞出现过度膨胀变圆的裂解式死亡现象,且EV71感染可诱导线粒体膜崩解、细胞膜损伤,提示病毒感染可能诱导多种细胞程序性死亡。为确认EV71感染诱导细胞程序性死亡的分子生物学特征,对EV71感染后细胞凋亡信号通路相关蛋白活化及其剪切进行蛋白免疫印迹分析,发现病毒感染可激活细胞凋亡通路。Gasdermin D是细胞焦亡信号通路的关键执行蛋白。邵峰课题组证明,被剪切的Gasdermin D的N端短片段足以诱导细胞焦亡发生[16];而细胞坏死性凋亡通常与RIPK1和RIPK3磷酸化及MLKL的磷酸化激活有关[17]。本研究对EV71感染后Gasdermin D和MLKL的表达及激活进行分析,未发现Gasdermin D和MLKL的表达水平有明显变化,也没有检测到其激活。结果表明,EV71感染诱导的细胞程序性死亡可能主要以细胞凋亡为主。在病毒感染前利用泛caspase抑制剂预处理细胞,观察细胞死亡,发现细胞凋亡相关蛋白caspase-9和PARP的激活被抑制后,细胞程序性死亡也明显被抑制,表明EV71感染诱导的细胞程序性死亡主要由caspase介导。LDH的释放可作为指示细胞膜完整性的标志[14],也是细胞凋亡区别于其他形式细胞程序性死亡的重要指标。LDH释放分析也获得类似结果,提示EV71感染主要诱导细胞凋亡,并依赖caspase的激活。
由于EV71感染后细胞形态出现多样性的病变,提示EV71感染可能诱导多种细胞程序性死亡方式。有文献报道EV71可激活caspase-1,诱导白细胞介素1β(interleukin 1β,IL-1β)、IL-18的表达,提示EV71感染可能诱导细胞焦亡[18-19]。但EV71感染后未检测到细胞焦亡效应蛋白Gasdermin D表达水平的明显改变和剪切激活,也没有发现坏死性凋亡信号通路的关键蛋白MLKL的表达发生明显变化及磷酸化激活,这或许与EV71诱导细胞焦亡或坏死性凋亡的水平较低有关。由于EV71感染主要诱导细胞凋亡,发生其他形式程序性死亡的细胞数量较少,蛋白免疫印迹法很难检测到。或许发展单细胞水平检测手段能全面了解EV71感染诱导细胞程序性死亡的特征。当然,EV71感染所激活的其他细胞程序性死亡也可能不依赖Gasdermin D和MLKL介导,而与其他替代途径相关。
综上所述,本研究初步表明EV71感染可诱导细胞程序性死亡,且以细胞凋亡为主,还可能低水平诱导其他形式细胞程序性死亡。对EV71感染诱导细胞程序性死亡及其可能分子机制的研究,有利于加深对EV71感染及其致病过程的理解,为研究EV71抗病毒药物、缓解EV71感染症状具有重要的理论和实际意义。
| [1] |
Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71[J]. Lancet Infect Dis, 2010, 10(11): 778-790.
[DOI]
|
| [2] |
Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71[J]. Lancet Neurol, 2010, 9(11): 1097-1105.
[DOI]
|
| [3] |
Weng KF, Chen LL, Huang PN, Shih SR. Neural pathogenesis of enterovirus 71 infection[J]. Microbes Infect, 2010, 12(7): 505-510.
[DOI]
|
| [4] |
Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection[J]. Nat Rev Immunol, 2017, 17(3): 151-164.
[DOI]
|
| [5] |
Nagata S, Tanaka M. Programmed cell death and the immune system[J]. Nat Rev Immunol, 2017, 17(5): 333-340.
[DOI]
|
| [6] |
Friedrich A, Pechstein J, Berens C, Lührmann A. Modulation of host cell apoptotic pathways by intracellular pathogens[J]. Curr Opin Microbiol, 2017, 35: 88-99.
[DOI]
|
| [7] |
Chan FK, Luz NF, Moriwaki K. Programmed necrosis in the cross talk of cell death and inflammation[J]. Annu Rev Immunol, 2015, 33: 79-106.
[DOI]
|
| [8] |
Aglietti RA, Dueber EC. Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions[J]. Trends Immunol, 2017, 38(4): 261-271.
[DOI]
|
| [9] |
Upton JW, Shubina M, Balachandran S. RIPK3-driven cell death during virus infections[J]. Immunol Rev, 2017, 277(1): 90-101.
[DOI]
|
| [10] |
Orzalli MH, Kagan JC. Apoptosis and necroptosis as host defense strategies to prevent viral infection[J]. Trends Cell Biol, 2017, 27(11): 800-809.
[DOI]
|
| [11] |
Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases[J]. Immunol Rev, 2017, 277(1): 61-75.
[DOI]
|
| [12] |
Simbulan-Rosenthal CM, Rosenthal DS, Iyer S, Boulares AH, Smulson ME. Transient poly(ADP-ribosyl)ation of nuclear proteins and role of poly(ADP-ribose) polymerase in the early stages of apoptosis[J]. J Biol Chem, 1998, 273(22): 13703-13712.
|
| [13] |
Wallberg F, Tenev T, Meier P. Analysis of apoptosis and necroptosis by fluorescence-activated cell sorting[J]. Cold Spring Harb Protoc, 2016.
[DOI]
|
| [14] |
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin[J]. Nature, 2017, 547(7661): 99-103.
[DOI]
|
| [15] |
Harris KG, Coyne CB. Death waits for no man-does it wait for a virus? How enteroviruses induce and control cell death[J]. Cytokine Growth Factor Rev, 2014, 25(5): 587-596.
[DOI]
|
| [16] |
Shi J, Gao W, Shao F. Pyroptosis:gasdermin-mediated programmed necrotic cell death[J]. Trends Biochem Sci, 2017, 42(4): 245-254.
[DOI]
|
| [17] |
He S, Huang S, Shen Z. Biomarkers for the detection of necroptosis[J]. Cell Mol Life Sci, 2016, 73(11-12): 2177-2181.
[DOI]
|
| [18] |
Yogarajah T, Ong KC, Perera D, Wong KT. AIM2 inflammasome-mediated pyroptosis in enterovirus A71-infected neuronal cells restricts viral replication[J]. Sci Rep, 2017, 7(1): 5845.
[DOI]
|
| [19] |
Wang Y, Qin Y, Wang T, Chen Y, Lang X, Zheng J, Gao S, Chen S, Zhong X, Mu Y, Wu X, Zhang F, Zhao W, Zhong Z. Pyroptosis induced by enterovirus 71 and coxsackievirus B3 infection affects viral replication and host response[J]. Sci Rep, 2018, 8(1): 2887.
[DOI]
|
 2018, Vol. 13
2018, Vol. 13


