肠道菌群被称为人体“第二基因组”,与其生活环境一起构成了人体内最复杂的微生态系统[1]。新生儿出生后,从母体和周围环境中获得各种微生物并进行肠道定植,建立肠道微生态系统并发挥重要生理功能,如保护、代谢调节、营养肠壁、维护肠道屏障和免疫调节等作用[2-3]。正常微生物群中细菌的数量最多,人肠道菌群中细菌数量高达100万亿个,重约2 kg[4]。近年来,随着高通量测序技术的不断发展,越来越多的证据表明肠道微生物群对人类的健康至关重要,其紊乱或失衡与许多疾病的发生发展密切相关,如炎症性肠病(inflammatory bowel disease,IBD)、糖尿病、肥胖、代谢综合征和炎症[5]。目前,抗生素滥用导致的不良反应越来越多,而益生菌的使用可减少这些不良反应,为人类治疗和改善疾病带来了新的希望。在已确定的二代益生菌中,嗜黏蛋白阿克曼菌(Akkermansia muciniphila,A. muciniphila)是有希望的候选者。事实上,其与肥胖、糖尿病、心脏代谢疾病和低度炎症呈负相关[6]。本文综述了A. muciniphila与相关疾病的研究进展。
1 A. muciniphila简介A. muciniphila隶属疣微菌门,是一种革兰阴性杆菌[7],2004年被Muriel Derrien首次分离鉴定。它是人类肠道微生物中最丰富的单物种之一,占细菌总量的0.5%~5.0%。A. muciniphila编码大量的黏蛋白降解酶,可降解黏液中的黏蛋白,产生短链脂肪酸,如丁酸盐、丙酸盐、乙酸盐等,在宿主的代谢和疾病中发挥作用[8-9]。A. muciniphila被称为降黏专家,以黏蛋白作为其唯一的碳和氮来源,专一性降解肠道黏蛋白,并刺激机体产生更多新的黏蛋白,导致黏液层增厚[10]; 其还能促进宿主天然免疫和获得性免疫系统的发育,具有抗炎作用[11]。目前已证实,肠道菌群中A. muciniphila的丰度除与肥胖、2型糖尿病(type 2 diabetes,T2DM)相关外,与动脉粥样硬化、阑尾炎、自闭症等也有密切关系[12]。因此,A. muciniphila被认为是有治疗意义的二代益生菌。
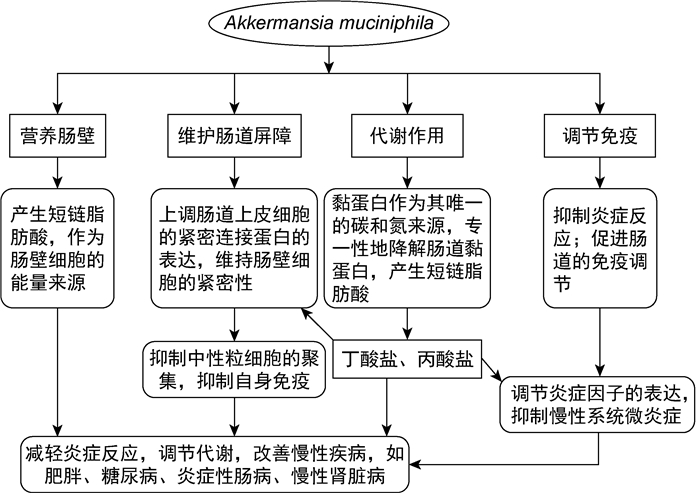
|
| 图 1 A. muciniphila的生理功能 Fig. 1 Physiological functions of A. muciniphila |
肥胖症是指体内脂肪堆积过多或分布异常而导致体重增加,包括遗传和环境因素在内的多种因素相互作用引起的慢性代谢性疾病,与多种疾病密切相关。肥胖症及其相关疾病可损害身心健康,降低生活质量,缩短寿命,已成为全球性公共卫生问题。肥胖患者体内存在严重的菌群失调。对10 534名来自美国和英国的20~99岁参与者的研究显示,体重指数和肥胖风险的增加与A. muciniphila相对丰度降低相关(r=0.03,P < 0.001),肥胖人群中A. muciniphila丰度低于健康人群,而高饱和脂肪酸饮食会导致A. muciniphila的丰度降低[13]。
肥胖状态下脂肪组织的病理性大规模扩张与低度炎症的发生有关,表现为细胞因子、趋化因子和促炎性脂肪酸的生成增强,导致白细胞产生的促炎因子与抗炎因子之间不平衡,从而进一步促进炎症发生和脂肪组织功能障碍(如β -氧化、褐变过程、脂肪生成),而肠道通透性增加成为代谢性炎症发生的关键[14]。A. muciniphila可通过调节炎性因子的产生改善高脂肪饮食引起的小鼠全身性炎症。研究表明,经A. muciniphila干预的高脂肪饮食小鼠肠道中促炎因子肿瘤坏死因子α (tumor necrosis factor α,TNF- α)、白细胞介素6(interleukin 6,IL-6)、单核细胞趋化蛋白1(monocyte chemoattractant protein-1,MCP-1)和Toll样受体2(Toll-like receptor 2,TLR-2)的表达水平显著降低,而抗炎因子白细胞介素10(interleukin 10,IL-10)的表达水平显著升高[15-16]。在小鼠中,A. muciniphila可提高抗炎因子α -生育酚和β -谷甾醇的全身浓度,其产生的耐热蛋白还可通过激活TLR-2和TLR-4而介导抗肥胖和抗糖尿病作用[17]。
研究表明,近1%的肠道上皮由具有肠道激素分泌特性的神经内分泌细胞组成,通过配体在L细胞中诱导G蛋白偶联受体(G protein-coupled receptor,GPCR)导致肽和激素[如胰高血糖素样肽1(glucagon-like peptide-1,GLP-1)]产生。GLP-1参与的宿主代谢包括食欲控制、参与葡萄糖和脂肪代谢、肠屏障功能、胃排空和肠运动。A. muciniphila产生的乙酸盐和丙酸盐作为共生微生物的营养物质,可通过诱导肠道L细胞中GPCR43和GPCR41的表达促进GLP-1的分泌,从而调节代谢[18]。因此,A. muciniphila被认为是人体代谢的有益参与者,具有治疗肥胖相关代谢紊乱的巨大前景。
2.2 A. muciniphila与T2DM糖尿病是常见的内分泌代谢性疾病,具有遗传易感性,由环境因素触发。近年来,随着人们饮食习惯和生活方式的改变,日益加重的T2DM负担成为全球关注的问题,而肥胖、肠道微生态紊乱是其主要病因。越来越多的研究表明,T2DM患者体内存在严重的菌群失调,重要益生菌A. muciniphila、拟杆菌、双歧杆菌的丰度显著降低[19]。其中拟杆菌、双歧杆菌具有耐氧性,甚至能兼性生长,而A. muciniphila是专性厌氧菌。将81例T2DM患者分为DP组(高纤维、富含多酚和植物蛋白功能性食品)和P组(安慰剂),进行为期12周的安慰剂对照随机双盲研究。结果表明,与P组相比,DP组普拉梭菌(Faecalibacterium prausnitzii,F. prausnitzii)增加了约34%,A. muciniphila增加了约125%,这两种细菌具有抗炎作用; 与P组相比,DP组患者血糖、总胆固醇、低密度脂蛋白胆固醇、游离脂肪酸、糖化血红蛋白、三酰甘油和C反应蛋白的曲线下面积显著降低,血清抗氧化活性增强[20]。
T2DM的特点是胰岛素水平较低、嗜黏液性、低度炎症和肠道通透性被破坏[21]。A. muciniphila可通过以下机制改善糖尿病。①抑制炎症反应:通过与TLR2结合的外膜蛋白改善肠道屏障功能,增强肠道屏障的局部作用,显著减少中性粒细胞浸润,从而减轻自身免疫炎症反应[22]。②抑制肠道内源性大麻素水平:内源性大麻素系统活性的增加已成为内脏肥胖的致病因素,而内脏肥胖是T2DM的危险因素。内源性大麻素系统由大麻素受体1型(type 1 cannabinoid receptor,CB1R)和大麻素受体2型(type 1 cannabinoid receptor,CB2R)2种GPCR组成。CB1R活性下调能缓解内质网和线粒体应激,这为治疗肥胖诱导的胰岛素抵抗和T2DM提供了新的思路[23]。16S rRNA宏基因组测序显示,在CB1R被阻断的小鼠粪便中,A. muciniphila的相对丰度显著增加,表明阻断CB1R可降低炎性因子和血浆脂多糖(lipopolysaccharide,LPS)的水平,降低肠道通透性,改善高血糖相关症状和提高胰岛素敏感性[24]。③产生有益代谢产物:A. muciniphila衍生聚糖产生的上皮定位短链脂肪酸丁酸盐是结肠细胞的首选能源,也是一种信号分子,可与肠上皮细胞和免疫细胞表面的特异性GPCR(如GPCR41)结合,调节促炎因子(如IL-18)的分泌; 通过GLP-1和肽酪氨酸-酪氨酸作用于中枢神经系统,调节食物摄入和能量消耗; 还可在免疫细胞和脂肪细胞中作为组蛋白去乙酰化酶抑制剂,通过染色质状态调节这些细胞的转录[25-26]。
胆汁酸(bile acid,BA)可调节血糖。BA通过核受体——法尼酯X受体(farnesoid X receptor,FXR)与GPCR5结合,参与三酰甘油合成、能量代谢调节和葡萄糖稳态调节。人成纤维细胞生长因子19(fibroblast growth factor 19,FGF19)在啮齿类动物中被称为FGF15,也在肠道中产生,是BA代谢的重要调节因子。A. muciniphila可通过调节β -CDCA的水平刺激胰岛素分泌和FGF19表达,从而降低血糖水平和调节葡萄糖稳态[27-28],是一种能改善血糖的潜在益生菌。
2.3 A. muciniphila与IBDIBD主要包括溃疡性结肠炎(ulcerative colitis,UC)和克罗恩病(Crohn’s disease,CD)。IBD患者中微生物群变化和细菌多样性降低与产丁酸菌丧失有关[29]。针对43例UC和57例CD患者的调查显示,与健康人群相比,UC和CD患者中A. muciniphila的定植和丰度显著降低[30]。
IBD(尤其CD)由肠道微生物群的异常黏膜免疫反应所致,通常由T细胞驱动,与促炎因子如TNF和干扰素γ (interferon γ,IFN- γ)增加有关[31]。在葡聚糖硫酸钠盐(dextran sulfate sodium salt,DSS)诱导的慢性结肠炎小鼠中,A. muciniphila具有抗炎作用,可改善脾脏重量、结肠炎症指数和结肠组织学评分等,还能下调TNF- α和IFN- γ等促炎因子的表达,从而促进肠道菌群正常化[32]。总之,A. muciniphila对IBD的保护机制在于其可抑制炎性因子TNF-a以及HT-29细胞分泌的炎性因子IL-8的分泌,从而调节免疫分化。
A. muciniphila对IBD的保护作用还表现在其有益代谢产物短链脂肪酸丁酸盐可激活肠道上皮细胞炎症体并促进抗炎因子IL-18的产生,而IL-18参与黏蛋白和抗菌肽的产生并控制肠道微生物群组成。同时,丁酸盐可抑制肠干细胞增殖,减少IBD患者隐窝及其表面上皮中的中性粒细胞数量,调节紧密连接蛋白的表达,从而促进肠屏障功能[31-33]。
2.4 A. muciniphila与慢性肾脏病慢性肾脏病(chronic kidney disease,CKD)是威胁人类健康的主要疾病之一,持续进展及心脑血管并发症是其导致死亡的主要原因。研究发现,CKD微炎症是疾病进展和并发症发生的独立危险因素[34],而肠道微生态失调是其主要诱因[35]。高通量测序结果表明,与健康对照者相比,CKD患者肠道微生态多样性和菌群结构存在明显的差异,A. muciniphila丰度显著降低(0.67% vs. 3.08%)[36]。最近研究表明,CKD患者由于肠上皮屏障中紧密连接蛋白ZO家族被破坏,肠道渗透性增加,导致内毒素和活细菌从肠腔转移到血流中,引发慢性炎症,从而增加了心血管风险和尿毒症毒性,加速了疾病进展[37]。因此,A. muciniphila不仅可调节肠道菌群的平衡,维护肠道屏障功能,还具有免疫调节效应。但补充A. muciniphila是否可通过维护肠道屏障功能和免疫调节作用改善肾脏病,尚未见报道。
2.5 A. muciniphila与其他疾病A. muciniphila的丰度与脂肪代谢呈极显著相关,A. muciniphila可抵抗高脂血症[38]。Wu和Li[39]研究发现,高酒精产力的肺炎克雷伯杆菌可通过产生内源性酒精诱导线粒体损伤,并破坏肠屏障,促使Th17等免疫细胞增殖,从而加剧肝脏炎症。而A. muciniphila可抑制肠道屏障通透性增加和内毒素移位,减少脂肪因子异常合成,减少炎性因子IL-6产生,还可调节肠道BA和FXR-FGF15轴的平衡,降低三酰甘油的水平,从而改善代谢紊乱相关性脂肪肝[40-41]。有研究表明,A. muciniphila可改善阿尔兹海默症小鼠大脑的淀粉样变性和斑块局限性神经炎症,减缓病理进程,缓解病变对小鼠空间学习记忆能力及认知能力的损害[42]。此外,A. muciniphila还可能通过改善肠道菌群、上调脑源性神经营养因子的水平、抑制海马神经炎症,从而缓解慢性应激诱导的小鼠抑郁样行为[43]。
3 结语综上所述,肠道微生物群在人类健康和疾病中有重要作用,肠道菌群中的A. muciniphila与肥胖症、T2DM、IBD、CKD等疾病的发生和进展密切相关,但具体机制尚不清楚。此外,除口服益生菌和粪便移植外,如何有效增加肠道中A. muciniphila的定植也须进一步探索。随着人们对A. muciniphila的研究不断深入,相信其作为潜在二代益生菌,在不久的将来能为代谢性疾病、肝损伤、IBD、肠道肿瘤等疾病的预防及治疗提供新的方向和选择。
| [1] |
Zhu B, Wang X, Li L. Human gut microbiome: the second genome of human body[J]. Protein Cell, 2010, 1(8): 718-725.
[DOI]
|
| [2] |
Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora[J]. Kidney Int, 2013, 83(2): 308-315.
[DOI]
|
| [3] |
Bose T, Venkatesh KV, Mande SS. Investigating host-bacterial interactions among enteric pathogens[J]. BMC Genomics, 2019, 20(1): 1022.
[DOI]
|
| [4] |
Biedermann L, Rogler G. The intestinal microbiota: its role in health and disease[J]. Eur J Pediatr, 2015, 174(2): 151-167.
[DOI]
|
| [5] |
Taguer M, Maurice CF. The complex interplay of diet, xenobiotics, and microbial metabolism in the gut: implications for clinical outcomes[J]. Clin Pharmacol Ther, 2016, 99(6): 588-599.
[DOI]
|
| [6] |
Cheng D, Xie MZ. A review of a potential and promising probiotic candidate—Akkermansia muciniphila[J]. J Appl Microbiol, 2021, 130(6): 1813-1822.
[DOI]
|
| [7] |
Davis JA, Collier F, Mohebbi M, Stuart AL, Loughman A, Pasco JA, Jacka FN. Obesity, Akkermansia muciniphila, and proton pump inhibitors: is there a link?[J]. Obes Res Clin Pract, 2020, 14(6): 524-530.
[DOI]
|
| [8] |
Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila[J]. Front Microbiol, 2017, 8: 1765.
[DOI]
|
| [9] |
Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W, Ning G, Liu R, Hong J. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice[J]. J Mol Endocrinol, 2017, 58(1): 1-14.
[DOI]
|
| [10] |
Frugé AD, Van der Pol W, Rogers LQ, Morrow CD, Tsuruta Y, Demark-Wahnefried W. Fecal Akkermansia muciniphila is associated with body composition and microbiota diversity in overweight and obese women with breast cancer participating in a presurgical weight loss trial[J]. J Acad Nutr Diet, 2020, 120(4): 650-659.
[DOI]
|
| [11] |
Xu Y, Wang N, Tan HY, Li S, Zhang C, Feng Y. Function of Akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems[J]. Front Microbiol, 2020, 11: 219.
[DOI]
|
| [12] |
提盼盼, 逄晓阳, 吕加平. 阿克曼粘细菌研究进展[J]. 食品工业科技, 2018, 39(13): 311-314. [CNKI]
|
| [13] |
Zhou Q, Zhang Y, Wang X, Yang RY, Zhu XQ, Zhang Y, Chen C, Yuan HP, Yang Z, Sun L. Gut bacteria Akkermansia is associated with reduced risk of obesity: evidence from the American Gut Project[J]. Nutr Metab (Lond), 2020, 17: 90.
[DOI]
|
| [14] |
Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice[J]. Sci Rep, 2015, 5: 16643.
[DOI]
|
| [15] |
Su Q. Phytochemicals in fenugreek seed prevent high fat diet induced metabolic inflammation and NAFLD via the mediation of Akkermansia muciniphila[J]. Proc Nutr Soc, 2020, 79(OCE2): E485.
[DOI]
|
| [16] |
Abuqwider JN, Mauriello G, Altamimi M. Akkermansia muciniphila, a new generation of beneficial microbiota in modulating obesity: a systematic review[J]. Microorganisms, 2021, 9(5): 1098.
[DOI]
|
| [17] |
Grajeda-Iglesias C, Durand S, Daillère R, Iribarren K, Lemaitre F, Derosa L, Aprahamian F, Bossut N, Nirmalathasan N, Madeo F, Zitvogel L, Kroemer G. Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites[J]. Aging (Albany NY), 2021, 13(5): 6375-6405.
|
| [18] |
Payahoo L, Khajebishak Y, Alivand MR, Soleimanzade H, Alipour S, Barzegari A, Ostadrahimi A. Investigation the effect of oleoylethanolamide supplementation on the abundance of Akkermansia muciniphila bacterium and the dietary intakes in people with obesity: A randomized clinical trial[J]. Appetite, 2019, 141: 104301.
[DOI]
|
| [19] |
Yang G, Wei J, Liu P, Zhang Q, Tian Y, Hou G, Meng L, Xin Y, Jiang X. Role of the gut microbiota in type 2 diabetes and related diseases[J]. Metabolism, 2021, 117: 154712.
[DOI]
|
| [20] |
Medina-Vera I, Sanchez-Tapia M, Noriega-López L, Granados-Portillo O, Guevara-Cruz M, Flores-López A, Avila-Nava A, Fernández ML, Tovar AR, Torres N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes[J]. Diabetes Metab, 2019, 45(2): 122-131.
[DOI]
|
| [21] |
Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, MICRO-Obes Consortium, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology[J]. Gut, 2016, 65(3): 426-436.
[DOI]
|
| [22] |
Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, Enrich B, Ciocan D, Macheiner S, Mayr L, Drach M, Moser P, Moschen AR, Perlemuter G, Szabo G, Cassard AM, Tilg H. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease[J]. Gut, 2018, 67(5): 891-901.
[DOI]
|
| [23] |
Lu D, Dopart R, Kendall DA. Controlled downregulation of the cannabinoid CB1 receptor provides a promising approach for the treatment of obesity and obesity-derived type 2 diabetes[J]. Cell Stress Chaperones, 2016, 21(1): 1-7.
[DOI]
|
| [24] |
Mehrpouya-Bahrami P, Chitrala KN, Ganewatta MS, Tang CB, Murphy EA, Enos RT, Velazquez KT, McCellan J, Nagarkatti M, Nagarkatti P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity[J]. Sci Rep, 2017, 7(1): 15645.
[DOI]
|
| [25] |
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites[J]. Cell, 2016, 165(6): 1332-1345.
[DOI]
|
| [26] |
Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, Deo A, Eid J, Gines J, Iyer M, Justice N, Loo WT, Nemchek M, Schicklberger M, Souza M, Stoneburner B, Tyagi S, Kolterman O. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation[J]. BMJ Open Diabetes Res Care, 2020, 8(1): e001319.
[DOI]
|
| [27] |
Zhang J, Ni YQ, Qian LL, Fang QC, Zheng TT, Zhang ML, Gao QM, Zhang Y, Ni JC, Hou XH, Bao YQ, Kovatcheva-Datchary P, Xu AM, Li HT, Panagiotou G, Jia W. Decreased abundance of Akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes[J]. Adv Sci (Weinh), 2021, e2100536.
[DOI]
|
| [28] |
Greer RL, Dong X, Moraes AC, Zielke RA, Fernandes GR, Peremyslova E, Vasquez-Perez S, Schoenborn AA, Gomes EP, Pereira AC, Ferreira SR, Yao M, Fuss IJ, Strober W, Sikora AE, Taylor GA, Gulati AS, Morgun A, Shulzhenko N. Akkermansia muciniphila mediates negative effects of IFNgamma on glucose metabolism[J]. Nat Commun, 2016, 7: 13329.
[DOI]
|
| [29] |
Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism[J]. Gut Microbes, 2016, 7(3): 189-200.
[DOI]
|
| [30] |
Zhang T, Li P, Wu X, Lu G, Marcella C, Ji X, Ji G, Zhang F. Alterations of Akkermansia muciniphila in the inflammatory bowel disease patients with washed microbiota transplantation[J]. Appl Microbiol Biotechnol, 2020, 104(23): 10203-10215.
[DOI]
|
| [31] |
Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease[J]. Nat Rev Immunol, 2009, 9(5): 313-323.
[DOI]
|
| [32] |
Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice[J]. Front Cell Infect Microbiol, 2019, 9: 239.
[DOI]
|
| [33] |
Gonçalves P, Araújo JR, Di Santo JP. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease[J]. Inflamm Bowel Dis, 2018, 24(3): 558-572.
[DOI]
|
| [34] |
Ruiz-Andres O, Sanchez-Niño MD, Moreno JA, Ruiz-Ortega M, Ramos AM, Sanz AB, Ortiz A. Downregulation of kidney protective factors by inflammation: role oftranscription factors and epigenetic mechanisms[J]. Am J Physiol Renal Physiol, 2016, 311(6): F1329-F1340.
[DOI]
|
| [35] |
Swift O, Vilar E, Farrington K. Unexplained inflammation in end-stage kidney disease: is the combination of enhanced gastrointestinal permeability and reticuloendothelial dysfunction its cause?[J]. Semin Dial, 2019, 32(5): 417-423.
[DOI]
|
| [36] |
Li F, Wang M, Wang J, Li R, Zhang Y. Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease[J]. Front Cell Infect Microbiol, 2019, 9: 206.
[DOI]
|
| [37] |
Sabatino A, Regolisti G, Brusasco I, Cabassi A, Morabito S, Fiaccadori E. Alterations of intestinal barrier and microbiota in chronic kidney disease[J]. Nephrol Dial Transplant, 2015, 30(6): 924-933.
[DOI]
|
| [38] |
Yu Y, Lu J, Sun L, Lyu XK, Chang XY, Mi X, Hu MG, Wu CM, Chen X. Akkermansia muciniphila: a potential novel mechanism of nuciferine to improve hyperlipidemia[J]. Biomed Pharmacother, 2021, 133: 111014.
[DOI]
|
| [39] |
Wu W, Li L. Protective effects of akkermansia muciniphila against non-alcoholic fatty liver disease through modulation of gut microbiota[J]. J Hepatol, 2020, 73: S241-S242.
[PubMed]
|
| [40] |
Kim S, Lee Y, Kim Y, Seo Y, Lee H, Ha J, Lee J, Choi Y, Oh H, Yoon Y. Akkermansia muciniphila prevents fatty liver, decreases serum triglycerides, and maintains gut homeostasis[J]. Appl Environ Microbiol, 2020, 86(7): e03004-19.
[DOI]
|
| [41] |
刘利敏, 姚明解, 胡端敏. 嗜黏蛋白阿克曼氏菌与肝损伤关系的研究进展[J]. 临床肝胆病杂志, 2020, 36(9): 223-226. [CNKI]
|
| [42] |
Chen Y, Fang L, Chen S, Zhou H, Fan Y, Lin L, Li J, Xu J, Chen Y, Ma Y, Chen Y. Gut microbiome alterations precede cerebral amyloidosis and microglial pathology in a mouse model of alzheimer's disease[J]. Biomed Res Int, 2020, 2020: 8456596.
[DOI]
|
| [43] |
Cheng RR, Xu WJ, Wang JC, Tang ZQ, Zhang M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila alleviates the depression-like behavior of depressed mice induced by chronic stress[J]. Biochem Biophys Res Commun, 2021, 566: 170-176.
[DOI]
|
 2022, Vol. 17
2022, Vol. 17



