2. 首都医科大学附属北京朝阳医院医学研究中心, 北京 100020
2. Medical Research Center, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, China
鲍曼不动杆菌是一种条件致病菌,在临床上常引发肺炎、败血症、泌尿系感染等机会性感染,在免疫抑制和重症监护室(intensive care unit,ICU)患者中较多见[1]。近年来,随着抗生素的滥用,出现了高度耐药鲍曼不动杆菌,给临床治疗带来了极大困难[2]。研究显示,鲍曼不动杆菌引发的宿主炎症反应不仅与宿主炎症免疫反应调节相关,还与菌体及其毒力因子诱导的细胞自噬紊乱相关[3-4]。许多病原体常因干扰了自噬体与溶酶体的融合,导致自噬降解清除失败,细胞自噬紊乱,从而造成不可控的炎症反应[5-6]。突触体相关蛋白29(synaptosomal-associated protein 29,SNAP29)在调节自噬溶酶体降解的过程中发挥关键作用。SNAP29是SNARE蛋白复合体的重要组成部分,是控制自噬体与溶酶体融合的关键分子,可介导自噬体膜表面的突触融合蛋白17(syntaxin 17,STX17)与溶酶体膜表面的囊泡相关膜蛋白8(vesicle-associated membrane protein 8,VAMP8)相连接。SNAP29的表达或功能失衡往往会引起自噬体与溶酶体融合失败,引发自噬降解障碍[7]。但到目前为止,鲍曼不动杆菌引起的自噬降解障碍是否与SNAP29相关尚不清楚。
此外,下游靶蛋白的表达和功能往往受上游调控因子的调控。研究发现,长链非编码RNA(long non-coding RNA,LncRNA)不仅能与下游功能蛋白相互作用,还能调控这些蛋白的功能[8-9]。其中,LncRNA-GAS5(生长抑制特异性转录本5,growth arrest-specific transcript 5),在细胞增殖、凋亡、自噬等生物学过程及各种疾病发展中发挥了重要作用[10-12]。本课题组前期研究证实,鲍曼不动杆菌可引起HeLa细胞中LncRNA-GAS5表达的增加,STX17表达减少,导致细胞自噬功能紊乱,从而引起小鼠肺损伤[13],但其是否通过LncRNA-GAS5影响SNAP29的表达而发挥调节作用仍未明晰。因此,本研究探讨了鲍曼不动杆菌对SNAP29蛋白表达的影响及LncRNA-GAS5在其中发挥的调节作用,从而为其引发的自噬紊乱的深层机制提供理论基础。
1 材料 1.1 菌株、细胞和表达载体鲍曼不动杆菌标准株ATCC 19606由本实验室保存;真核细胞表达载体pcDNA3.1和敲减载体pRNAT-U6.1/Neo购自美国Addgene公司;人宫颈癌细胞HeLa细胞由本实验室保存。
1.2 主要仪器和试剂主要仪器和试剂如下:NehI/XhoI、BamHI/HindIII限制性内切酶(美国NEB公司);质粒大提试剂盒HiPure Plasmid MaxiPrep Kit、凝胶回收试剂盒、First-Stand cDNA Synthesis SuperMix反转录试剂盒(北京全式金生物技术股份有限公司);LB培养基、DMEM培养基、胎牛血清、青/链霉素(美国Gibco公司);SNAP29(ab138500)抗体(Abcam公司);GAPDH(#5174)抗体(美国CST公司);聚合酶链反应(polymerase chain reaction,PCR)仪(ABI9700,美国ABI公司);核酸和蛋白电泳仪、电泳槽、凝胶成像系统(美国BioRad公司);Odyseey红外荧光成像系统(美国LI-COR公司)。
2 方法 2.1 鲍曼不动杆菌制备及HeLa细胞培养采用三分区划线法将鲍曼不动杆菌涂布于血培养皿,于37 ℃温箱培养,待形成可见菌落后,用接种环挑取单个菌落,接种于3 mL LB液体培养基,37 ℃、180 r/min摇床过夜。然后,用分光光度计检测菌液光密度(optical density,OD),用无菌磷酸盐缓冲液(phosphate buffered saline,PBS)调节菌液密度至1×106CFU/mL,用于后续细胞感染实验。HeLa细胞用含10%胎牛血清和1%青/链霉素的DMEM培养基于37 ℃、5% CO2细胞培养箱中培养。
2.2 鲍曼不动杆菌感染HeLa细胞将HeLa细胞以1×106 CFU/mL的密度接种于6孔板,用DMEM完全培养基培养24 h。然后,将鲍曼不动杆菌按感染复数(multiplicity of infection,MOI)为10感染HeLa细胞,于不同时间点收集细胞,用于蛋白免疫印迹检测。
2.3 过表达载体pcDNA3.1-GAS5的构建通过PCR方法扩增LncRNA-GAS5基因,将扩增产物和pcDNA3.1(+)载体分别用NehI和XhoI双酶切,回收LncRNA-GAS5和pcDNA3.1 (+)载体,用T4连接酶(北京TaKaRa公司)于16 ℃连接30 min,转化大肠埃希菌感受态细胞,用PCR鉴定阳性克隆,送至苏州泓讯生物科技股份有限公司进行测序。
2.4 敲减载体pRNAT-U6-GAS5的构建采用Block-iTTM RNAi Designer软件设计3对敲减序列,针对短发夹RNA(short hairpin RNA,shRNA)靶点,设计并合成寡核苷酸(oligonucleotide,Oligo),将合成好的Oligo进行退火处理。用BamHI和HindIII对pRNAT-U6.1/Neo进行酶切回收,通过T4 DNA连接酶将经退火处理的Oligo与pRNAT-U6.1/Neo进行连接。
重组反应条件:Oligo退火产物4 μ L,酶切后的pRNAT-U6.1/Neo 1 μ L,10×T4 DNA Ligase Buffer 2 μ L,T4 DNA Ligase 1 μ L,PEG 4000 2 μ L,加入ddH2O调整总体积至20 μ L,22 ℃反应2 h。
最后,转化大肠埃希菌感受态细胞并进行阳性克隆鉴定及测序分析,扩大培养,提取高纯度质粒用于后续细胞转染实验。将构建好的敲减质粒分别命名为sh-GAS5-1、sh-GAS5-2、sh-GAS5-3,空载质粒命名为sh-NC。
2.5 重组质粒转染HeLa细胞及表达鉴定将pcDNA3.1-GAS5、pRNAT-U6.1-GAS5分别转染HeLa细胞48 h,用TRIzol溶解细胞,采用氯仿-异丙醇法提取细胞总RNA,用反转录试剂盒First-Stand cDNA Synthesis SuperMix将RNA反转录为cDNA。实时荧光定量PCR(real-time fluorescence quantitative PCR,qPCR)反应体系如下:2×qPCR Super Mix 10 μ L、Passive Reference Dye 0.4 μ L、正反向引物各0.4 μ L,补ddH2O调至总体积20 μ L。最后,通过Real-Time PCR(ABI 7500)检测LncRNA-GAS5的表达,采用2-ΔΔCt分析法计算基因表达的相对比值。
2.6 引物本研究所用引物如表 1所示。
| Methods | Primer name | Sequence (5′-3′) | Product size (bp) |
| PCR | GAS5-F | TATGTCTTTTCGAGGTAGGAGTC | 668 |
| GAS5-R | TAGGATGGATTGCAAAAATTT | ||
| RNA interference | GAS5-sh-1 | GGCUCUGGAUAGCACCUUA | |
| GAS5-sh-2 | GGAUGAGAAUAGCUACUGA | ||
| GAS5-sh-3 | GACCUGUUAUCCUAAACUA | ||
| qPCR | GAS5-F | TGGTTCTGCTCCTGGTAACG | 185 |
| GAS5-R | AGGATAACAGGTCTGCCTGC | ||
| GAPDH-F | GGAGCGAGATCCCTCCAAAAT | 197 | |
| GAPDH-R | GGCTGTTGTCATACTTCTCATGG |
收集转染过表达质粒或敲减质粒48 h后的HeLa细胞,PBS洗3次,用RIPA裂解液于冰上裂解5 min,12 000 g离心10 min,收集蛋白上清备用。用BCA试剂盒检测蛋白浓度。进行10%十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(sodium dodecyl sulfate-polyacrylamide gel electrophoresis,SDS-PAGE)后,将凝胶上的分离蛋白转移至0.22 μ m聚偏氟乙烯(polyvinylidene fluoride,PVDF)膜(美国Merck Millipore公司),用5%脱脂奶粉室温封闭1 h。裁下目的条带,与SNAP29或GAPDH于4 ℃孵育过夜,用1×TBST洗膜3次,然后与山羊抗兔荧光标记二抗(1∶15 000稀释)室温孵育1 h,再用1×TBST洗膜3次,用Odyssey红外荧光成像系统检测蛋白表达,用Image J分析灰度值。
2.8 统计学分析所有实验数据均以mean±SEM形式表示,采用Prism 5.0软件(GraphPad Software Inc. San Diego,CA)作图,SPSS 17.0软件进行统计学分析,行t检验,P < 0.05为有统计学差异。
3 结果 3.1 LncRNA-GAS5在HeLa细胞中表达的鉴定为鉴定LncRNA-GAS5过表达质粒是否在HeLa细胞中发挥作用,本研究采用qPCR检测pcDNA3.1-GAS5转染HeLa细胞48 h后GAS5的表达量。结果显示,过表达质粒转染HeLa细胞48 h后GAS5大量表达,与空载体组相比有显著性差异(P < 0.01,见图 1)。

|
| **P < 0.01 compared with pcDNA3.1 group. 图 1 LncRNA-GAS5在HeLa细胞中过表达 Fig. 1 Overexpression of LncRNA-GAS5 in HeLa cells |
为鉴定pRNAT-U6.1-GAS5的敲减效率,本研究采用qPCR检测转染敲减质粒48 h后HeLa细胞中GAS5的表达量。结果显示,敲减质粒sh-GAS5-1敲减的HeLa细胞中GAS5的表达量降至未敲减细胞的53%(P < 0.05),sh-GAS5-2组降至未敲减细胞的75%,sh-GAS5-3组降至未敲减细胞的35%。提示sh-GAS5-3敲减质粒能显著抑制HeLa细胞中GAS5的表达量,敲减效率最高,与空质粒转染组比较差异显著(P < 0.01,见图 2)。因此,后续实验采用sh-GAS5-3质粒进行敲减。

|
| *P < 0.05, **P < 0.01 compared with sh-NC group. 图 2 LncRNA-GAS在HeLa细胞中被敲减 Fig. 2 Knockdown of LncRNA-GAS5 in HeLa cells |
为探究LncRNA-GAS5对SNAP29表达的影响,本研究采用蛋白免疫印迹法检测过表达和敲减GAS5后HeLa细胞中SNAP29的表达量。结果显示,过表达GAS5可使SNAP29表达显著减少,与pcDNA3.1空质粒转染组相比差异显著(P < 0.01,见图 3);而敲减GAS5后SNAP29表达水平显著升高,与sh-NC空质粒转染组相比差异显著(P < 0.01,见图 3)。
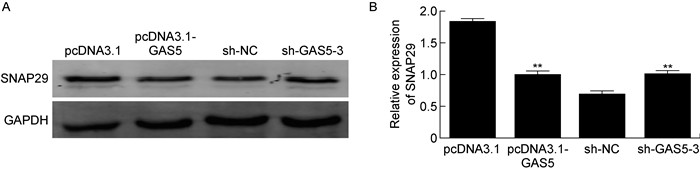
|
|
A: The effect of LncRNA-GAS5 on the expression of SNAP29 detected by Western blotting. B: Quantitative analysis of SNAP29 expression. **P < 0.01 compared with pcDNA3.1 or sh-NC group. 图 3 LncRNA-GAS5对HeLa细胞中SNAP29表达的影响 Fig. 3 Effect of LncRNA-GAS5 on the expression of SNAP29 in HeLa cells |
用鲍曼不动杆菌(MOI=10)感染HeLa细胞2 h、4 h、8 h、16 h、24 h后,蛋白免疫印迹检测的结果显示,随着鲍曼不动杆菌感染时间的延长,SNAP29的表达不断降低,具有时间依赖性,与未感染组相比有显著的差异(P < 0.01,见图 4A)。用sh-GAS5-3质粒敲减GAS5后再用鲍曼不动杆菌感染HeLa细胞4 h,蛋白免疫印迹检测的结果显示,与sh-NC+鲍曼不动杆菌组比较,SNAP29的表达量显著恢复(P < 0.01,见图 4B)。pcDNA3.1-GAS5过表达48 h后再用鲍曼不动杆菌感染HeLa细胞4 h,SNAP29的表达被进一步抑制,与pcDNA3.1+鲍曼不动杆菌组比较,具有统计学差异(P < 0.01,见图 4C)。
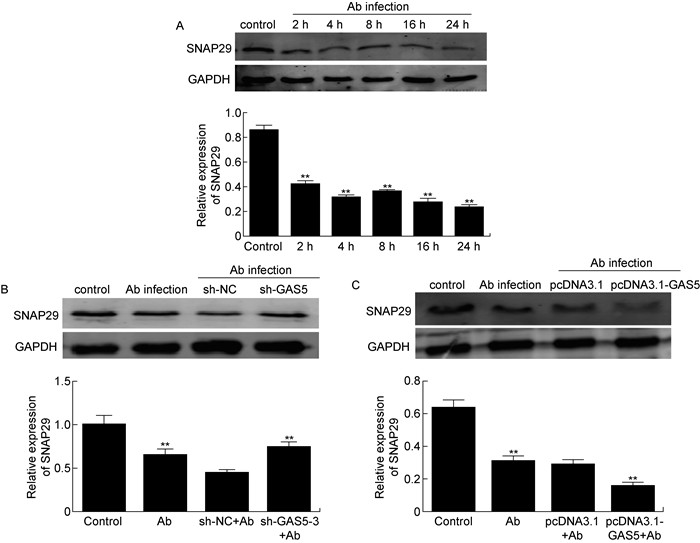
|
|
A:Western blotting was used to detect the expression of SNAP29 after Acinetobacter baumannii infection at different times. B: Western blotting was used to detect the effect of Acinetobacter baumannii infection on the expression of SNAP29 after knockdown of GAS5. C: The effect of Acinetobacter baumannii infection on the expression of SNAP29 after overexpression of GAS5 was detected by Western blotting. The data is represented as mean±SEM (n=3). * *P < 0.01 compared with the control group. Acinetobacter baumannii is abbreviated as Ab. 图 4 LncRNA-GAS5调节鲍曼不动杆菌对SNAP29的降解 Fig. 4 Degradation of SNAP29 by Acinetobacter baumannii regulated by LncRNA-GAS5 |
本研究成功构建了pcDNA3.1-GAS5过表达载体和pRNAT-U6.1-GAS5敲减载体,并使其在HeLa细胞中高效表达,从而发现LncRNA-GAS5对SNAP29的表达具有负向调节作用。鲍曼不动杆菌可降解HeLa细胞中的SNAP29,此降解作用受到LncRNA-GAS5的调节,证明鲍曼不动杆菌对SNAP29的降解依赖于LncRNA-GAS5,进一步解释了鲍曼不动杆菌引起细胞自噬降解障碍的机制。
众所周知,LncRNA通过多种方式调控下游自噬基因和蛋白的表达,涉及转录前、转录、翻译等多维度调控[14]。例如:LncRNA H19通过表观遗传沉默DIRAS3抑制自噬[15];LncRNA-CAIF(心肌自噬抑制因子,cardiac autophagy inhibitory factor)通过阻断p53介导的心肌蛋白转录抑制来自噬和减轻心肌梗死[16]。本研究初步发现,LncRNA-GAS5可调控SNAP29的表达,但还不清楚这一调控作用于哪个层面,具体机制较为复杂,有待进一步研究。
细菌或病毒通过调控LncRNA来调节靶分子的表达,从而有效促进自身存活。Salerno等[17]发现,乙型肝炎病毒X蛋白(hepatitis B virus x protein,HBx)可与淋巴细胞白血病缺失基因2(deleted in lymphocytic leukemia 2,LncRNA-DLEU2)结合来维持其共价闭合环状DNA(covalently closed circular DNA,cccDNA)的复制和提高宿主癌基因转录。丙型肝炎病毒可提高肝癌高表达转录本(highly up-regulated in liver cancer,LncRNA-HULC)的表达,从而使其核心蛋白有效绑定至细胞膜脂筏上以促进病毒颗粒的释放[18]。相反,宿主也会调动自身的LncRNA调控免疫防御系统以控制病原菌感染。例如,LncRNA-MEG3(母系表达基因3,maternally expressed gene 3)可通过控制MiR-145-5p表达来调节巨噬细胞的增殖以控制结核分枝杆菌感染[19]。本研究中鲍曼不动杆菌通过LncRNA-GAS5来调节SNAP29的表达,敲除LncRNA-GAS5可使SNAP29的表达增加,而过表达LncRNA-GAS5则可使SNAP29表达进一步受到抑制。
综上所述,鲍曼不动杆菌通过LncRNA-GAS5调节SNAP29的表达可能是其引起自噬降解障碍的重要原因,或许可通过调节LncRNA-GAS5来控制SNAP29的表达以提高宿主的自噬降解效率,从而控制鲍曼不动杆菌感染。
| [1] |
Vázquez-López R, Solano-Gálvez SG, Juárez Vignon-Whaley JJ, Abello Vaamonde JA, Padró Alonzo LA, Rivera Reséndiz A, Muleiro Álvarez M, Vega López EN, Franyuti-Kelly G, Álvarez-Hernández DA, Moncaleano Guzmán V, Juárez Bañuelos JE, Marcos Felix J, González Barrios JA, Barrientos Fortes T. Acinetobacter baumannii resistance: a real challenge for clinicians[J]. Antibiotics (Basel), 2020, 9(4): 205.
[DOI]
|
| [2] |
Kurihara MNL, Sales RO, Silva KED, Maciel WG, Simionatto S. Multidrug-resistant Acinetobacter baumannii outbreaks: a global problem in healthcare settings[J]. Rev Soc Bras Med Trop, 2020, 53: e20200248.
[DOI]
|
| [3] |
Parra-Millán R, Guerrero-Gómez D, Ayerbe-Algaba R, Pachón-Ibáñez ME, Miranda-Vizuete A, Pachón J, Smani Y. Intracellular trafficking and persistence of Acinetobacter baumannii requires transcription factor EB[J]. mSphere, 2018, 3(2): e00106-18.
[DOI]
|
| [4] |
Rumbo C, Tomás M, Fernández Moreira E, Soares NC, Carvajal M, Santillana E, Beceiro A, Romero A, Bou G. The Acinetobacter baumannii Omp33-36 porin is a virulence factor that induces apoptosis and modulates autophagy in human cells[J]. Infect Immun, 2014, 82(11): 4666-4680.
[DOI]
|
| [5] |
Ding B, Zhang G, Yang X, Zhang S, Chen L, Yan Q, Xu M, Banerjee AK, Chen M. Phosphoprotein of human parainfluenza virus type 3 blocks autophagosome-lysosome fusion to increase virus production[J]. Cell Host Microbe, 2014, 15(5): 564-577.
[DOI]
|
| [6] |
Chen X, Wang K, Xing Y, Tu J, Yang X, Zhao Q, Li K, Chen Z. Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity[J]. Protein Cell, 2014, 5(12): 912-927.
[DOI]
|
| [7] |
Lő rincz P, Juhász G. Autophagosome-Lysosome fusion[J]. J Mol Biol, 2020, 432(8): 2462-2482.
[DOI]
|
| [8] |
Sauvageau M. Diverging RNPs: toward understanding lncRNA-protein interactions and functions[J]. Adv Exp Med Biol, 2019, 1203: 285-312.
[PubMed]
|
| [9] |
Ferrè F, Colantoni A, Helmer-Citterich M. Revealing protein-lncRNA interaction[J]. Brief Bioinform, 2016, 17(1): 106-116.
[DOI]
|
| [10] |
Zhou Y, Chen B. GAS5 mediated regulation of cell signaling[J]. Mol Med Rep, 2020, 22(4): 3049-3056.
[PubMed]
|
| [11] |
Jiang X, Ning Q. The mechanisms of lncRNA GAS5 in cardiovascular cells and its potential as novel therapeutic target[J]. J Drug Target, 2020, 28(10): 1012-1017.
[DOI]
|
| [12] |
Yang X, Xie Z, Lei X, Gan R. Long non-coding RNA GAS5 in human cancer[J]. Oncol Lett, 2020, 20(3): 2587-2594.
[PubMed]
|
| [13] |
An Z, Ding W. .Acinetobacter baumannii up-regulates LncRNA-GAS5 and promotes the degradation of STX17 by blocking the activation of YY1[J]. Virulence, 2021, 12(1): 1965-1979.
|
| [14] |
Yang L, Wang H, Shen Q, Feng L, Jin H. Long non-coding RNAs involved in autophagy regulation[J]. Cell Death Dis, 2017, 8(10): e3073.
[DOI]
|
| [15] |
Zhuo C, Jiang R, Lin X, Shao M. LncRNA H19 inhibits autophagy by epigenetically silencing of DIRAS3 in diabetic cardiomyopathy[J]. Oncotarget, 2017, 8(1): 1429-1437.
|
| [16] |
Liu CY, Zhang YH, Li RB, Zhou LY, An T, Zhang RC, Zhai M, Huang Y, Yan KW, Dong YH, Ponnusamy M, Shan C, Xu S, Wang Q, Zhang YH, Zhang J, Wang K. LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription[J]. Nat Commun, 2018, 9(1): 29.
[DOI]
|
| [17] |
Salerno D, Chiodo L, Alfano V, Floriot O, Cottone G, Paturel A, Pallocca M, Plissonnier ML, Jeddari S, Belloni L, Zeisel M, Levrero M, Guerrieri F. Hepatitis B protein HBx binds the DLEU2 lncRNA to sustain cccDNA and host cancer-related gene transcription[J]. Gut, 2020, 69(11): 2016-2024.
|
| [18] |
Sharma G, Tripathi SK, Das S. lncRNA HULC facilitates efficient loading of HCV-core protein onto lipid droplets and subsequent virus-particle release[J]. Cell Microbiol, 2019, 21(10): e13086.
[DOI]
|
| [19] |
Sun W, Lou H, Cao J, Wang P, Sha W, Sun Q. LncRNA MEG3 control Mycobacterium Tuberculosis infection via controlled MiR-145-5p expression and modulation of macrophages proliferation[J]. Microb Pathog, 2020, 149: 104550.
[DOI]
|
 2022, Vol. 17
2022, Vol. 17


