2. 上海交通大学医学院免疫学与微生物学系,上海 200025
2. Department of Immunology and Microbiology, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China
外膜囊泡(outer membrane vesicle,OMV)是革兰阴性菌在各种特殊刺激条件下分泌至细菌胞外、直径为10 nm~400 nm的球状脂质双层颗粒,其主要成分包括细菌蛋白、核酸、毒素等[1]。在革兰阴性菌生长各阶段均可见到OMV以不对称的小泡或囊泡形式分泌至细菌表面及胞外环境中[2],但其组成和种类可因细菌种类、菌株特征、培养条件以及细菌生长阶段的不同而产生差异[3]。
一般认为OMV起源于革兰阴性菌的细胞膜,因此各种影响胞膜稳定性的因素均可导致OMV分泌量增加[4],如异常高温或低温、低pH值、缺氧环境、营养条件、抗生素等外界压力,它们既可通过内源性途径激活细菌应激反应或前噬菌体而引起细胞膜稳定性下降,也可直接破坏细胞膜成分,使OMV分泌量明显增加[5]。在OMV产生过程中,革兰阴性菌外膜成分,如脂质、蛋白、脂多糖(lipopolysaccharide,LPS)以及胞质内的部分遗传物质和蛋白质,均可被包装进OMV,进而发挥生理作用[6-7]。该过程的具体机制尚未明确,但其有助于革兰阴性菌耐受恶劣环境。OMV可引起人体局部或全身慢性炎症和细胞损伤,这可能是临床使用抗生素治疗革兰阴性菌感染的不良反应之一。
广谱抗生素会破坏人体正常微生物群而导致微生态紊乱,从而引发一系列非感染性疾病,这已引起临床医师重视。人体微生物群特别是肠道微生物群暴露于抗生素后,除了一些人体正常的敏感性细菌被杀死而导致微生态失衡以外,大量细菌在抗生素作用下释放OMV也可能是引起临床非感染性疾病的主要因素之一。根据作用机制,抗生素可被分为3类:蛋白质合成抑制剂、核酸合成抑制剂及作用于细胞膜和细胞壁的抗生素。它们均可引起各种革兰阴性菌分泌OMV,但分泌机制及类型不同,本文对此进行综述。
1 蛋白质合成抑制剂类抗生素氨基糖苷类、四环素类等抗生素主要与细菌核糖体结合,抑制其蛋白质合成,进而起到抑菌作用。它们可破坏革兰阴性菌细胞壁中的脂多糖(lipopolysaccharide, LPS)和细菌表面蛋白等结构,促使OMV释放。此类OMV可介导感染部位的慢性炎症反应、细菌耐药及机体组织细胞损伤等。
1.1 氨基糖苷类抗生素与铜绿假单胞菌OMV介导的慢性炎症反应庆大霉素是典型的氨基糖苷类抗生素,不仅可与细菌30S亚基结合,抑制其蛋白质合成,还可替代Ca2+、Mg2+等阳离子结合到细菌外膜LPS,改变LPS结构,破坏外膜稳定性,最终引起OMV释放[8](见图 1AⅠ)。有研究表明,庆大霉素可使铜绿假单胞菌的OMV释放量增加3~5倍[9],但OMV引发慢性炎症的机制尚未明确。有研究认为,铜绿假单胞菌OMV中的鞭毛蛋白、亚硝酸盐还原酶和LPS等可刺激免疫细胞产生以白细胞介素8(interleukin-8,IL-8)为主的趋化因子,介导中性粒细胞募集和激活,从而引起感染部位的慢性炎症[10](见图 1CⅠ)。但另一项研究发现,铜绿假单胞菌OMV所含的短链RNA(short RNA,sRNA)能够靶向宿主mRNA,抑制IL-8等细胞因子分泌和中性粒细胞浸润,降低宿主对其的免疫应答[11](见图 1CⅡ)。这两种机制可能在铜绿假单胞菌感染所引起的慢性肺炎中发挥了综合作用,有待进一步研究。
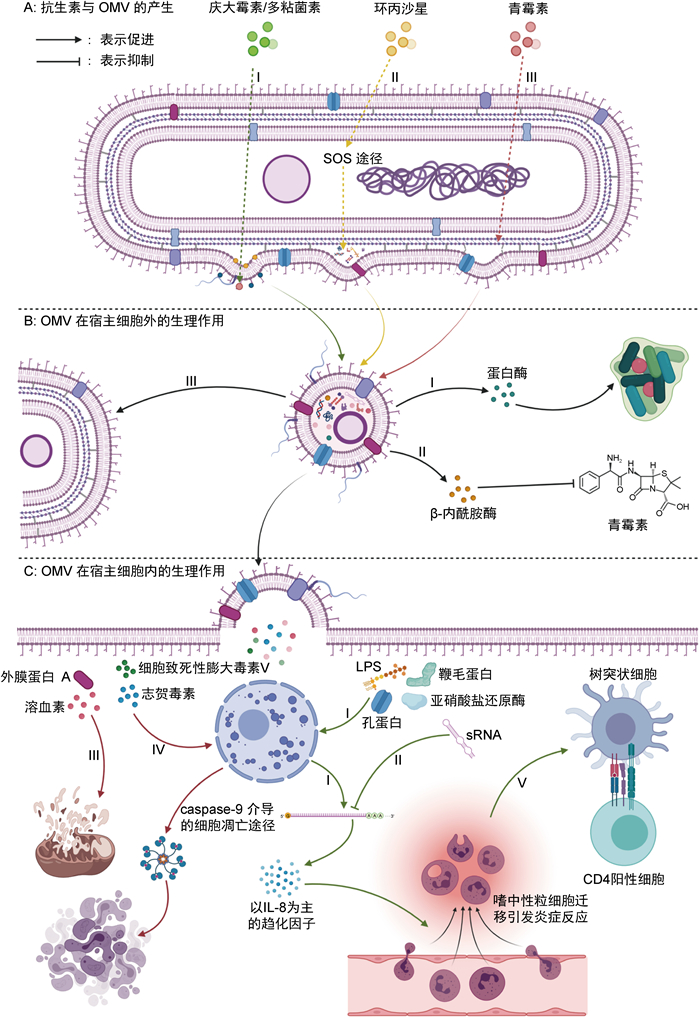
|
| A:3种不同类型抗生素引起OMV分泌的机制。Ⅰ:庆大霉素和多黏菌素破坏LPS,外膜稳定性降低;Ⅱ:在环丙沙星作用下,细菌SOS机制激活,导致胞质周围间隙内细菌代谢废物局部积累,对外膜施加局部膨胀压力;Ⅲ:青霉素作用于肽聚糖层,使肽聚糖与外膜之间的脂蛋白交联局部减少。B:OMV在细胞外发挥生理作用的机制。Ⅰ:OMV中的蛋白酶可破坏宿主组织结构,形成生物膜,以便于细菌生长繁殖;Ⅱ:OMV中的β-内酰胺酶可分解环境中的青霉素,增强细菌群落对β-内酰胺类抗生素的耐药性;Ⅲ:OMV可以极高的效率将质粒转移至其他革兰阴性菌。C:OMV在细胞内发挥作用的机制。Ⅰ:OMV中的鞭毛蛋白、亚硝酸盐还原酶、LPS和孔蛋白等可刺激免疫细胞产生IL-8等趋化因子,引起中性粒细胞募集和炎症反应;Ⅱ:OMV中的sRNA能够靶向宿主mRNA,抑制IL-8等细胞因子分泌和中性粒细胞浸润;Ⅲ:OMV中的外膜蛋白A和溶血素可损伤线粒体,最终引起细胞死亡;Ⅳ:OMV中的志贺毒素、细胞致死性膨胀毒素V可导致细胞DNA损伤,通过caspase-9途径介导细胞凋亡;Ⅴ:OMV刺激感染部位形成炎症后,可导致树突状细胞趋化及细胞免疫强烈激活。 图 1 抗生素诱导革兰阴性菌产生OMV的途径及发挥生理功能的机制 Fig. 1 Biogenesis and physiological functions of outer membrane vesicles triggered by antibiotics in gram-negative bacteria |
铜绿假单胞菌OMV还可通过改变细菌生存环境引起机体慢性炎症。其富含的各类蛋白酶可破坏宿主组织结构,使细菌更牢固地黏附于宿主表面,形成生物膜,以便细菌生长繁殖[12](见图 1BⅠ)。此外,铜绿假单胞菌可通过OMV向环境中释放自溶素,裂解其他细菌(如大肠埃希菌)的肽聚糖(peptidoglycan,PG)层以发挥杀菌作用[13]。
1.2 四环素类抗生素与鲍曼不动杆菌OMV的分泌及致病机制依拉环素是一类新型含氟四环素类抗生素,适用于多重耐药菌引起的严重感染。最近研究发现,鲍曼不动杆菌暴露于依拉环素后,其OMV分泌大量增加,且OMV的蛋白组成发生了改变,但其具体机制尚未明确[14]。诸多研究发现,鲍曼不动杆菌OMV的多种成分在其致病过程中起关键作用,其中外膜蛋白A(outer membrane protein A,OmpA)是导致炎症反应和细胞损伤的重要因素之一[15]。OmpA可激活宿主的GTPase动力相关蛋白,引起线粒体断裂、活性氧产生增加和细胞死亡[16](见图 1CⅢ)。除OmpA外,鲍曼不动杆菌OMV的孔蛋白可使其在免疫和结缔组织细胞中持续存在并产生细胞毒性[17]。OMV还可通过Toll样受体(Toll-like receptor,TLR)途径介导肺部炎症,引起在中性粒细胞聚集感染部位[18]。另有报道认为,鲍曼不动杆菌可通过OMV将自身的β-内酰胺酶基因转移至其他细菌(如大肠埃希菌或其他种类鲍曼不动杆菌),从而增加受体菌株对β-内酰胺类抗生素的抗性[19]。
2 核酸合成抑制剂类抗生素喹诺酮类、硝咪唑类、新生霉素等抗生素可抑制细菌DNA合成相关的酶,或直接损伤细菌DNA,从而发挥杀菌作用。此类抗生素可能还会激活细菌内RecA依赖的SOS应激反应,导致OMV分泌增加及成分改变,造成严重的宿主细胞损伤或感染加重。
2.1 喹诺酮类抗生素与大肠埃希菌OMV介导的细胞毒性环丙沙星是合成的第三代喹诺酮类药物,具有广谱抗菌活性,是目前临床常用抗生素之一。但研究发现,环丙沙星可刺激肠出血型大肠埃希菌(enterohemorrhagic Escherichia coli,EHEC)、铜绿假单胞菌、嗜麦芽窄食单胞菌等革兰阴性菌分泌大量OMV。这可能是环丙沙星激活了细菌内RecA依赖的SOS应激反应,导致受损或折叠错误的蛋白质等代谢废物在胞质周围间隙中局部积累,并不断对外膜施加局部膨胀压力,最终外膜外凸形成OMV。其具体机制尚未明确,有待进一步探讨[20](见图 1AⅡ)。
EHEC在环丙沙星刺激下所分泌的OMV含有溶血素、志贺毒素、细胞致死性膨胀毒素V等致病因子,其中溶血素可损伤线粒体并破坏其膜电位(见图 1CⅢ),而志贺毒素、细胞致死性膨胀毒素V可引起DNA损伤,通过caspase-9介导的细胞凋亡途径诱导细胞周期停滞,并最终导致细胞凋亡[21](见图 1CⅣ)。环丙沙星对EHEC的刺激还上调了OMV中志贺毒素2a的水平,其随OMV进入血液循环后可引起溶血性尿毒症综合征和心功能不全等[22-23]。此外,大肠埃希菌OMV的外膜蛋白会诱导嗜中性粒细胞表达趋化因子CXCL1,促进嗜中性粒细胞向肺组织迁移,介导急性肺损伤[24]。某些大肠埃希菌产生的OMV还能与环境中的多黏菌素类抗生素结合并将其降解,从而保护自身及菌群中的其他细菌[25]。甚至,某些抗生素的抗性基因可通过OMV转移至其他种类大肠埃希菌中,而环丙沙星的刺激可显著增强这一过程[26]。
除大肠埃希菌外,环丙沙星的刺激还可促使嗜麦芽窄食单胞菌分泌OMV,增加其细胞毒性和抗生素耐药性[27]。
2.2 作用于DNA的抗生素与志贺菌OMV疫苗丝裂霉素C(mitomycin C,MMC)可直接作用于细菌DNA,从而抑制细菌合成,但其对正常细胞也有较强的毒性,故一般不用于细菌感染的常规治疗。与喹诺酮类抗生素相似,MMC在细菌内可引发RecA依赖的SOS应激反应,进而激活细菌内前噬菌体,通过编码内溶素破坏细菌PG层,从而降低外膜稳定性,产生OMV[28-29]。研究发现,与喹诺酮类抗生素不同的是,经MMC处理后,痢疾志贺菌的OMV释放量和OMV中志贺毒素含量均显著增加,但具体机制尚未明确[30]。
有趣的是,不同于其他革兰阴性菌OMV的致病作用,志贺菌OMV在体内更多地表现为保护性而非致病性。相关动物实验证实,通过皮下注射、口服等多种途径,OMV可刺激感染部位形成炎症,导致树突状细胞趋化及细胞免疫强烈激活,明显增强了小鼠对福氏志贺菌的免疫力[31](见图 1CⅤ)。
3 作用于细菌细胞膜和细胞壁的抗生素常见的细胞壁合成抑制剂类抗生素包括β-内酰胺类和糖肽类,细胞膜功能抑制剂类抗生素主要有多黏菌素类、多烯类及吡咯类。此类抗生素可直接破坏细菌细胞膜结构或抑制细菌细胞壁合成,引起结构异常,进而导致OMV释放。
3.1 β-内酰胺类抗生素与嗜麦芽窄食单胞菌OMV建立的群落抗生素抗性头孢菌素和青霉素在临床广泛应用,其通过与青霉素结合蛋白结合干扰细菌合成PG,破坏细菌细胞壁结构而发挥杀菌作用。与MMC激活细菌内前噬菌体后前噬菌体编码的内溶素破坏细菌PG层不同,此类抗生素可直接干扰细菌PG的合成,使细菌PG层与外膜层的交联减少,外膜相对过度延伸,进而形成OMV[32](见图 1AⅢ)。诸多研究证实,亚致死浓度的β-内酰胺类抗生素可诱导多种革兰阴性菌(如大肠埃希菌、肺炎克雷伯菌、鲍曼不动杆菌、洋葱伯克霍尔德菌、嗜麦芽窄食单胞菌等)分泌OMV并增强其对宿主细胞的损伤和致炎作用[33-36]。
以嗜麦芽窄食单胞菌为例,研究发现其OMV可刺激肺上皮细胞表达促炎细胞因子和趋化因子,造成肺上皮细胞损伤[37]。更重要的是,嗜麦芽窄食单胞菌常与其他种类的细菌(如铜绿假单胞菌、盲肠伯克霍尔德菌等)形成细菌群落,受青霉素刺激后其OMV分泌增加,而OMV中大量的β-内酰胺酶可有效分解环境中的青霉素,使细菌群落对β-内酰胺类抗生素的耐药性大大增强[33](见图 1BⅡ)。
3.2 多黏菌素类抗生素与肺炎克雷伯菌OMV介导的基因水平转移近年来革兰阴性菌对抗生素的耐药性增加,多黏菌素类已成为治疗多重耐药革兰阴性菌感染的最后一道防线[38]。但最近研究发现,与庆大霉素介导OMV产生的机制类似,多黏菌素类可与多种革兰阴性菌(如肺炎克雷伯菌、空肠弯曲杆菌、大肠埃希菌等)表面LPS的脂质A结合,破坏细菌外膜结构的完整性,进而促进OMV释放并上调OMV中部分脂质和蛋白的含量[39-40](见图 1AⅠ)。以肺炎克雷伯菌为例,研究发现其OMV中的LPS、孔蛋白和鞭毛蛋白等可强烈诱导支气管上皮细胞表达IL-6、IL-8和肿瘤坏死因子等炎症因子,进而导致大量中性粒细胞浸润和强烈的免疫反应[41-42](见图 1CⅠ)。对肺炎克雷伯菌OMV刺激的支气管上皮细胞进行基因表达分析,发现上皮细胞内除相关细胞因子的基因表达明显上调外,DNA损伤和细胞凋亡相关的基因表达也有所上调,证明肺炎克雷伯菌OMV对支气管上皮细胞同样存在毒性[43]。
此外,肺炎克雷伯菌通过OMV将质粒转移至其他革兰阴性菌(如大肠埃希菌、肠沙门菌和铜绿假单胞菌等)的效率极高,使不同细菌在较大范围内传递抗生素耐药基因成为可能[44](见图 1BⅢ)。不同于质粒,OMV介导的基因水平转移受环境因素的影响较小,基因转移更高效且稳定[45],这是因为OMV可借助膜脂质的流动性将基因转移至受体细胞内释放。
3.3 其他作用于细菌细胞壁的抗生素其他作用于细菌细胞壁的抗生素有糖肽类和磷霉素等。有研究称磷霉素、环丙沙星和多黏菌素类均可强烈刺激大肠埃希菌分泌OMV,但有关磷霉素的作用机制尚无报道[22]。有研究认为,糖肽类抗生素万古霉素可诱导粪肠球菌产生膜囊泡,但目前尚无糖肽类抗生素诱导革兰阴性菌产生OMV的报道[46]。
4 结语许多研究显示,抗生素治疗革兰阴性菌感染不仅会影响人体正常菌群导致菌群失调,还会刺激细菌产生大量OMV,而OMV不断刺激人体免疫系统,可引发感染部位的慢性炎症,这也是目前临床上使用抗生素治疗革兰阴性菌感染导致不良反应的机制之一。此外,OMV中的诸多毒力因子在某些特殊情况下可能大量进入宿主血液,引起严重的毒血症,甚至死亡。
早在1919年抗生素尚未被发现时,就有利用噬菌体治疗细菌感染的报道,但1929年后,随着越来越多的抗生素被发现并投入使用,噬菌体治疗的相关研究大幅减少。近年来,随着对人体微生物群功能认识的深入及抗生素耐药问题的日益严重,抗生素治疗引起的诸多不良反应越来越受重视。相比之下,噬菌体治疗的不良反应报道较少,这可能与其特异性靶向杀灭细菌有关。噬菌体在杀灭细菌的过程中可利用大部分细菌物质合成自身,从而在很大程度上减少了细菌内毒力物质的释放,降低了不良反应的发生率。目前,噬菌体在人体内与细菌及人体免疫系统的相互作用还有待进一步研究,但开发利用噬菌体靶向细菌的治疗是解决多重耐药菌感染及减少抗生素治疗不良反应的重要途径。
| [1] |
Schwechheimer C, Sullivan CJ, Kuehn MJ. Envelope control of outer membrane vesicle production in Gram-negative bacteria[J]. Biochemistry, 2013, 52(18): 3031-3040.
[DOI]
|
| [2] |
Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles[J]. Microbiol Mol Biol Rev, 2010, 74(1): 81-94.
[DOI]
|
| [3] |
Taheri N, Fällman M, Wai SN, Fahlgren A. Accumulation of virulence-associated proteins in Campylobacter jejuni outer membrane vesicles at human body temperature[J]. J Proteomics, 2019, 195: 33-40.
[DOI]
|
| [4] |
Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions[J]. Nat Rev Microbiol, 2015, 13(10): 605-619.
[DOI]
|
| [5] |
Klimentova J, Pavkova I, Horcickova L, Bavlovic J, Kofronova O, Benada O, Stulik J. Francisella tularensis subsp. holarctica releases differentially loaded outer membrane vesicles under various stress conditions[J]. Front Microbiol, 2019, 10: 2304.
[DOI]
|
| [6] |
Cahill BK, Seeley KW, Gutel D, Ellis TN. Klebsiella pneumoniae O antigen loss alters the outer membrane protein composition and the selective packaging of proteins into secreted outer membrane vesicles[J]. Microbiol Res, 2015, 180: 1-10.
[DOI]
|
| [7] |
Sjöström AE, Sandblad L, Uhlin BE, Wai SN. Membrane vesicle-mediated release of bacterial RNA[J]. Sci Rep, 2015, 5: 15329.
[DOI]
|
| [8] |
Kadurugamuwa JL, Clarke AJ, Beveridge TJ. Surface action of gentamicin on Pseudomonas aeruginosa[J]. J Bacteriol, 1993, 175(18): 5798-5805.
[DOI]
|
| [9] |
Kadurugamuwa JL, Beveridge TJ. Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release[J]. J Antimicrob Chemother, 1997, 40(5): 615-621.
[DOI]
|
| [10] |
Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response[J]. Microbes Infect, 2006, 8(9-10): 2400-2408.
[DOI]
|
| [11] |
Koeppen K, Hampton TH, Jarek M, Scharfe M, Gerber SA, Mielcarz DW, Demers EG, Dolben EL, Hammond JH, Hogan DA, Stanton BA. A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles[J]. PLoS Pathog, 2016, 12(6): e1005672.
[DOI]
|
| [12] |
Esoda CN, Kuehn MJ. Pseudomonas aeruginosa leucine aminopeptidase influences early biofilm composition and structure via vesicle-associated antibiofilm activity[J]. mBio, 2019, 10(6): e02548-19.
[DOI]
|
| [13] |
Clarke AJ. The "hole" story of predatory outer-membrane vesicles[J]. Can J Microbiol, 2018, 64(9): 589-599.
[DOI]
|
| [14] |
Kesavan D, Vasudevan A, Wu L, Chen J, Su Z, Wang S, Xu H. Integrative analysis of outer membrane vesicles proteomics and whole-cell transcriptome analysis of eravacycline induced Acinetobacter baumannii strains[J]. BMC Microbiol, 2020, 20(1): 31.
[DOI]
|
| [15] |
Skerniškytė J, Karazijaitė E, Lučiūnaitė A, Sužiedėlienė E. OmpA protein-deficient Acinetobacter baumannii outer membrane vesicles trigger reduced inflammatory response[J]. Pathogens, 2021, 10(4): 407.
[DOI]
|
| [16] |
Tiku V, Kofoed EM, Yan D, Kang J, Xu M, Reichelt M, Dikic I, Tan MW. Outer membrane vesicles containing OmpA induce mitochondrial fragmentation to promote pathogenesis of Acinetobacter baumannii[J]. Sci Rep, 2021, 11(1): 618.
[DOI]
|
| [17] |
Rumbo C, Tomás M, Fernández Moreira E, Soares NC, Carvajal M, Santillana E, Beceiro A, Romero A, Bou G. The Acinetobacter baumannii Omp33-36 porin is a virulence factor that induces apoptosis and modulates autophagy in human cells[J]. Infect Immun, 2014, 82(11): 4666-4680.
[DOI]
|
| [18] |
Marion CR, Lee J, Sharma L, Park KS, Lee C, Liu W, Liu P, Feng J, Gho YS, Dela Cruz CS. Toll-like receptors 2 and 4 modulate pulmonary inflammation and host factors mediated by outer membrane vesicles derived from Acinetobacter baumannii[J]. Infect Immun, 2019, 87(9): e00243-19.
[DOI]
|
| [19] |
Chatterjee S, Mondal A, Mitra S, Basu S. Acinetobacter baumannii transfers the blaNDM-1 gene via outer membrane vesicles[J]. J Antimicrob Chemother, 2017, 72(8): 2201-2207.
[DOI]
|
| [20] |
Maredia R, Devineni N, Lentz P, Dallo SF, Yu J, Guentzel N, Chambers J, Arulanandam B, Haskins WE, Weitao T. Vesiculation from Pseudomonas aeruginosa under SOS[J]. ScientificWorldJournal, 2012, 2012: 402919.
[DOI]
|
| [21] |
Bielaszewska M, Ruter C, Bauwens A, Greune L, Jarosch KA, Steil D, Zhang W, He X, Lloubes R, Fruth A, Kim KS, Schmidt MA, Dobrindt U, Mellmann A, Karch H. Host cell interactions of outer membrane vesicle-associated virulence factors of enterohemorrhagic Escherichia coli O157: Intracellular delivery, trafficking and mechanisms of cell injury[J]. PLoS Pathog, 2017, 13(2): e1006159.
[DOI]
|
| [22] |
Bauwens A, Kunsmann L, Karch H, Mellmann A, Bielaszewska M. Antibiotic-mediated modulations of outer membrane vesicles in enterohemorrhagic Escherichia coli O104: H4 and O157: H7[J]. Antimicrob Agents Chemother, 2017, 61(9): e00937-17.
[DOI]
|
| [23] |
Svennerholm K, Park KS, Wikström J, Lässer C, Crescitelli R, Shelke GV, Jang SC, Suzuki S, Bandeira E, Olofsson CS, Lötvall J. Escherichia coli outer membrane vesicles can contribute to sepsis induced cardiac dysfunction[J]. Sci Rep, 2017, 7(1): 17434.
[DOI]
|
| [24] |
Lee J, Yoon YJ, Kim JH, Dinh NTH, Go G, Tae S, Park KS, Park HT, Lee C, Roh TY, Di Vizio D, Gho YS. Outer membrane vesicles derived from Escherichia coli regulate neutrophil migration by induction of endothelial IL-8[J]. Front Microbiol, 2018, 9: 2268.
[DOI]
|
| [25] |
Kulkarni HM, Nagaraj R, Jagannadham MV. Protective role of E. coli outer membrane vesicles against antibiotics[J]. Microbiol Res, 2015, 181: 1-7.
[DOI]
|
| [26] |
Bielaszewska M, Daniel O, Karch H, Mellmann A. Dissemination of the blaCTX-M-15 gene among Enterobacteriaceae via outer membrane vesicles[J]. J Antimicrob Chemother, 2020, 75(9): 2442-2451.
[DOI]
|
| [27] |
Devos S, Van Putte W, Vitse J, Van Driessche G, Stremersch S, Van Den Broek W, Raemdonck K, Braeckmans K, Stahlberg H, Kudryashev M, Savvides SN, Devreese B. Membrane vesicle secretion and prophage induction in multidrug-resistant Stenotrophomonas maltophilia in response to ciprofloxacin stress[J]. Environ Microbiol, 2017, 19(10): 3930-3937.
[DOI]
|
| [28] |
Toyofuku M, Cárcamo-Oyarce G, Yamamoto T, Eisenstein F, Hsiao CC, Kurosawa M, Gademann K, Pilhofer M, Nomura N, Eberl L. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis[J]. Nat Commun, 2017, 8(1): 481.
[DOI]
|
| [29] |
Turnbull L, Toyofuku M, Hynen AL, Kurosawa M, Pessi G, Petty NK, Osvath SR, Cárcamo-Oyarce G, Gloag ES, Shimoni R, Omasits U, Ito S, Yap X, Monahan LG, Cavaliere R, Ahrens CH, Charles IG, Nomura N, Eberl L, Whitchurch CB. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms[J]. Nat Commun, 2016, 7: 11220.
[DOI]
|
| [30] |
Dutta S, Iida K, Takade A, Meno Y, Nair GB, Yoshida S. Release of Shiga toxin by membrane vesicles in Shigella dysenteriae serotype 1 strains and in vitro effects of antimicrobials on toxin production and release[J]. Microbiol Immunol, 2004, 48(12): 965-969.
[DOI]
|
| [31] |
Camacho AI, de Souza J, Sánchez-Gómez S, Pardo-Ros M, Irache JM, Gamazo C. Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice[J]. Vaccine, 2011, 29(46): 8222-8229.
[DOI]
|
| [32] |
Koning RI, de Breij A, Oostergetel GT, Nibbering PH, Koster AJ, Dijkshoorn L. Cryo-electron tomography analysis of membrane vesicles from Acinetobacter baumannii ATCC19606 T[J]. Res Microbiol, 2013, 164(5): 397-405.
[DOI]
|
| [33] |
Devos S, Stremersch S, Raemdonck K, Braeckmans K, Devreese B. Intra-and interspecies effects of outer membrane vesicles from Stenotrophomonas maltophilia on beta-lactam resistance[J]. Antimicrob Agents Chemother, 2016, 60(4): 2516-2518.
[DOI]
|
| [34] |
Ye C, Li W, Yang Y, Liu Q, Li S, Zheng P, Zheng X, Zhang Y, He J, Chen Y, Hua L, Yang Z, Li D, Ren Z, Yang Y, Qi J, Huang W, Ma Y. Inappropriate use of antibiotics exacerbates inflammation through OMV-induced pyroptosis in MDR Klebsiella pneumoniae infection[J]. Cell Rep, 2021, 36(12): 109750.
[DOI]
|
| [35] |
Michel LV, Gallardo L, Konovalova A, Bauer M, Jackson N, Zavorin M, McNamara C, Pierce J, Cheng S, Snyder E, Hellman J, Pichichero ME. Ampicillin triggers the release of Pal in toxic vesicles from Escherichia coli[J]. Int J Antimicrob Agents, 2020, 56(6): 106163.
[DOI]
|
| [36] |
Kim SY, Kim MH, Son JH, Kim SI, Yun SH, Kim K, Kim S, Shin M, Lee JC. Outer membrane vesicles produced by Burkholderia cepacia cultured with subinhibitory concentrations of ceftazidime enhance pro-inflammatory responses[J]. Virulence, 2020, 11(1): 995-1005.
[DOI]
|
| [37] |
Kim YJ, Jeon H, Na SH, Kwon HI, Selasi GN, Nicholas A, Park TI, Lee SH, Lee JC. Stenotrophomonas maltophilia outer membrane vesicles elicit a potent inflammatory response in vitro and in vivo[J]. Pathog Dis, 2016, 74(8): ftw104.
[DOI]
|
| [38] |
Velkov T, Roberts KD, Nation RL, Thompson PE, Li J. Pharmacology of polymyxins: new insights into an 'old' class of antibiotics[J]. Future Microbiol, 2013, 8(6): 711-724.
[DOI]
|
| [39] |
Jasim R, Han ML, Zhu Y, Hu X, Hussein MH, Lin YW, Zhou QT, Dong CYD, Li J, Velkov T. Lipidomic analysis of the outer membrane vesicles from paired polymyxin-susceptible and-resistant Klebsiella pneumoniae clinical isolates[J]. Int J Mol Sci, 2018, 19(8): 2356.
[DOI]
|
| [40] |
Godlewska R, Klim J, Debski J, Wyszynska A, Lasica A. Influence of environmental and genetic factors on proteomic profiling of outer membrane vesicles from Campylobacter jejuni[J]. Pol J Microbiol, 2019, 68(2): 255-261.
[DOI]
|
| [41] |
Lee JC, Lee EJ, Lee JH, Jun SH, Choi CW, Kim SI, Kang SS, Hyun S. Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune response[J]. FEMS microbiol lett, 2012, 331(1): 17-24.
[DOI]
|
| [42] |
Martora F, Pinto F, Folliero V, Cammarota M, Dell'Annunziata F, Squillaci G, Galdiero M, Morana A, Schiraldi C, Giovane A, Galdiero M, Franci G. Isolation, characterization and analysis of pro-inflammatory potential of Klebsiella pneumoniae outer membrane vesicles[J]. Microb Pathog, 2019, 136: 103719.
[DOI]
|
| [43] |
Dell'Annunziata F, Ilisso CP, Dell'Aversana C, Greco G, Coppola A, Martora F, Dal Piaz F, Donadio G, Falanga A, Galdiero M, Altucci L, Galdiero M, Porcelli M, Folliero V, Franci G. Outer membrane vesicles derived from Klebsiella pneumoniae influence the miRNA expression profile in human bronchial epithelial BEAS-2B cells[J]. Microorganisms, 2020, 8(12): 1985.
[DOI]
|
| [44] |
Dell'Annunziata F, Dell'Aversana C, Doti N, Donadio G, Dal Piaz F, Izzo V, De Filippis A, Galdiero M, Altucci L, Boccia G, Galdiero M, Folliero V, Franci G. Outer membrane vesicles derived from Klebsiella pneumoniae are a driving force for horizontal gene transfer[J]. Int J Mol Sci, 2021, 22(16): 8732.
[DOI]
|
| [45] |
Velimirov B, Ranftler C. Unexpected aspects in the dynamics of horizontal gene transfer of prokaryotes: the impact of outer membrane vesicles[J]. Wien Med Wochenschr, 2018, 168(11-12): 307-313.
[DOI]
|
| [46] |
Kim MH, Kim SY, Son JH, Kim SI, Lee H, Kim S, Shin M, Lee JC. Production of membrane vesicles by Enterococcus faecium cultured with or without subinhibitory concentrations of antibiotics and their pathological effects on epithelial cells[J]. Front Cell Infect Microbiol, 2019, 9: 295.
[DOI]
|
 2022, Vol. 17
2022, Vol. 17


